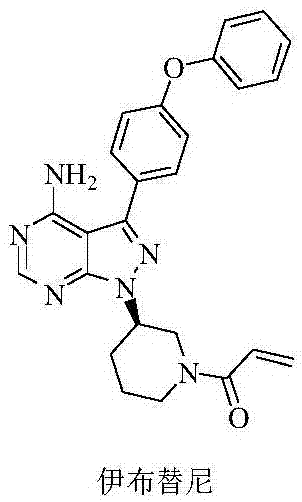
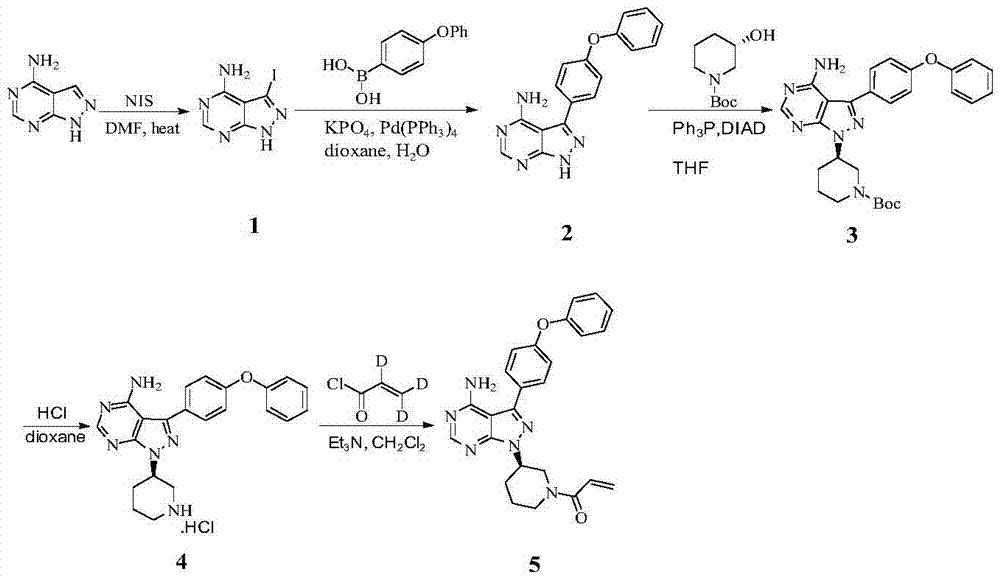
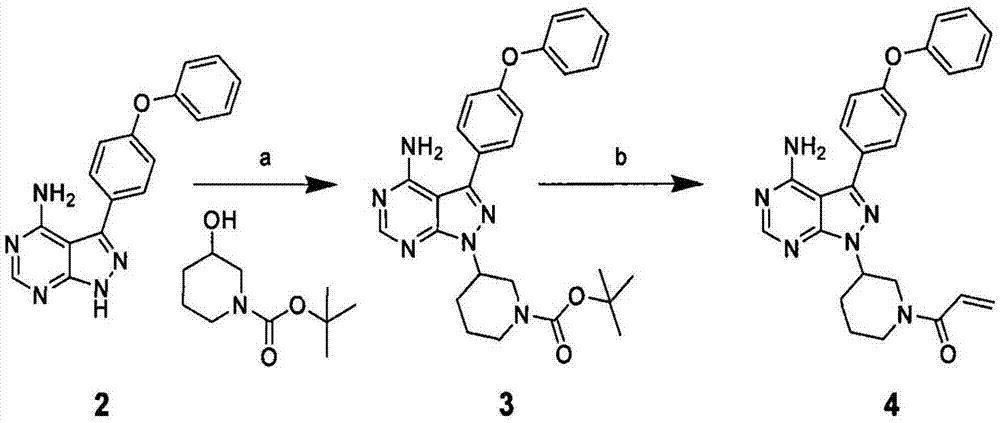
Preparation method of Bruton's tyrosine kinase inhibitor
A tyrosine kinase and inhibitor technology, which is applied in the field of drug synthesis, can solve the problems of high production cost, cumbersome steps, complicated processes, etc., and achieves the effects of low cost, concise steps, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Preparation of intermediate Ⅰ
[0036] Add 600g of (S)-tert-butyloxycarbonyl-3-hydroxypiperidine, 780g of triphenylphosphine and 3LDMF into the reaction flask and stir to dissolve, then add 300g of 4-amino-3-(4-benzene Oxyphenyl)-1H-pyrido(3,4-d)pyrimidine, continue to stir for 10-30min, avoid light, start adding diisopropyl azodicarboxylate dropwise at 10-15℃ under temperature control, drop After the addition is complete, raise the temperature to 25-30°C, stir and react for 3-4h; stop the reaction, cool down to 0°C, start to add 1110mL concentrated HCl dropwise, after the dropwise addition, warm up to room temperature, stir for 3-4h; stop the reaction, Add 3L of purified water and stir, then add 1.8L of chloroform for 5 times and 1.8L of ethyl acetate for 2 times. After the extraction, add 1.2L of ethyl acetate, cool down to 0°C, and start adding 25% NaOH solution dropwise , adjust the pH value to 8-9, a large amount of solids are precipitated, stirred and crystall...
Embodiment 2
[0040] 1. Preparation of intermediate Ⅰ
[0041] Add 600g of (S)-tert-butyloxycarbonyl-3-hydroxypiperidine, 780g of triphenylphosphine and 3L of acetone into the reaction flask and stir to dissolve, then add 300g of 4-amino-3-(4- Phenoxyphenyl)-1H-pyrido(3,4-d)pyrimidine, continue to stir for 10-30min, avoid light, start adding diisopropyl azodicarboxylate dropwise at 10-15°C under temperature control, After the dropwise addition, raise the temperature to 25-30°C, stir and react for 3-4h; stop the reaction, cool down to 0°C, start to add 1110mL concentrated HCl dropwise, after the dropwise addition, warm up to room temperature, stir for 3-4h; stop the reaction , add 3L of purified water and stir, then add 1.8L of chloroform for 5 times and 1.8L of ethyl acetate for 2 times, after the extraction, add 1.2L of ethyl acetate, cool down to 0°C, and start adding 25% NaOH dropwise solution, adjust the pH value to 8-9, precipitate a large amount of solids, stir and crystallize, the c...
Embodiment 3
[0045] 1. Preparation of intermediate Ⅰ
[0046] Add 600g of (S)-tert-butyloxycarbonyl-3-hydroxypiperidine, 780g of triphenylphosphine and 3L of ethyl acetate into the reaction flask and stir to dissolve, then add 300g of 4-amino-3-( 4-phenoxyphenyl)-1H-pyrido(3,4-d)pyrimidine, continue to stir for 10-30 minutes, keep away from light, and start adding diisopropyl azodicarboxylate dropwise at 10-15°C under temperature control Esters, after the dropwise addition, raise the temperature to 25-30°C, stir for 3-4 hours; stop the reaction, cool down to 0°C, start adding 1110mL of concentrated HCl dropwise, after the dropwise addition, warm up to room temperature, and stir for 3-4h; Stop the reaction, add 3L of purified water and stir, then add 1.8L of chloroform for 5 times and 1.8L of ethyl acetate for 2 times, after the extraction, add 1.2L of ethyl acetate, cool down to 0°C, and start adding 25 % NaOH solution, adjust the pH value to 8-9, a large amount of solids are precipitated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com