Method for suppressing ras activated in cell by using antibody having cytoplasm penetration capacity and complete immunoglobulin form, and use for same
An immunoglobulin and antibody technology, applied in the composition of preventing, treating or diagnosing cancer, treating cancer or tumor, and the antibody field comprising the variable region of the heavy chain, which can solve the problems of insufficient research, weak host resistance, fusion Problems such as low cell penetration efficiency of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0200] Example 1: Heavy chain variable binding specifically to GTP-bound KRas by a highly diverse human VH library Region (VH) to select
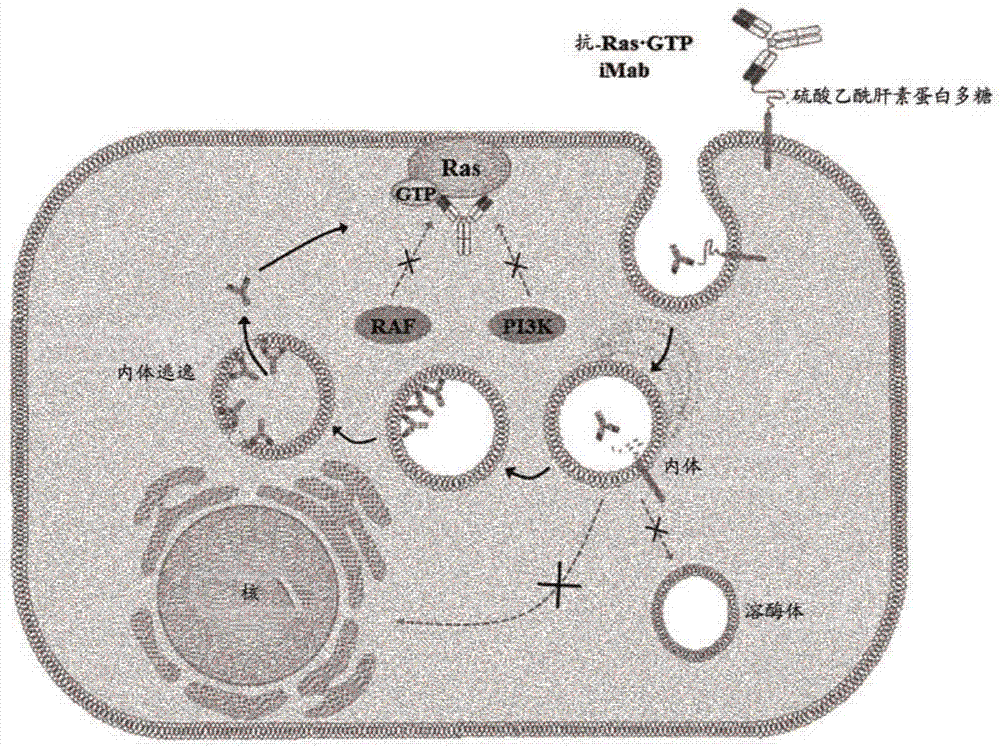
[0201] figure 1 A schematic diagram showing the strategy for inducing cytotoxicity specific for Ras mutant cells by using the following monoclonal antibody (Anti-Ras GTP iMab: Internalization and Interfering Monoclonal Antibody) by incorporating the heavy chain of IgG type cytotransmab The variable region (VH) (only capable of penetrating the cytosol) was constructed by replacing the variable region (VH) of the heavy chain that specifically binds to GTP-bound KRas and the antibody penetrated cells and remained in the cytosol Binds specifically to GTP-bound Ras.
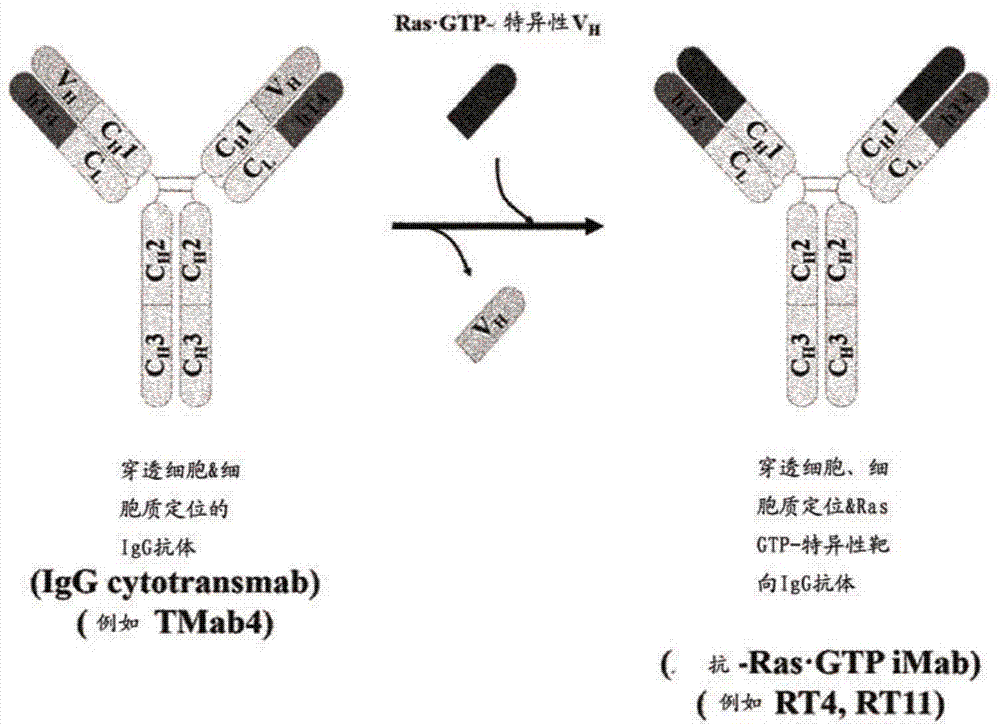
[0202] figure 2 A schematic diagram showing the construction by replacing the heavy chain variable region (VH) of an intact IgG-type cytotransmab capable of penetrating only the cytosol with a heavy chain variable region (VH) that specifically binds GTP-bound KRas Methods o...
Embodiment 2
[0205] Example 2: Preparation of GTP-bound KRas G12D protein
[0206] Expression and purification in E. coli for the preparation of GTP-bound KRas G12D antigen for library screening and affinity analysis is described in detail in a previously reported paper (Tanaka T et al., 2007).
[0207] Specifically, residues 1-188 of the CAAX motif comprising wild-type KRas and mutants KRas G12D, KRas G12V, and KRas G13D (listed with higher to lower mutation frequencies) were identified by using the restriction enzymes BamHI / EcoRI. The coding DNA was cloned into the Escherichia coli expression vector pGEX-3X. In this application, the expression vector was designed with T7 promoter-GST-KRas. All KRas mutations were induced using overlapping PCR techniques and expression vectors were constructed using the methods described above. The pGEX-3X-KRas vector was transformed into E. coli by electroporation and selected in selection medium. The selected Escherichia coli were cultured in LB me...
Embodiment 3
[0209] Example 3: Selection of the heavy chain variable region (VH) specific for GTP-bound KRas G12D
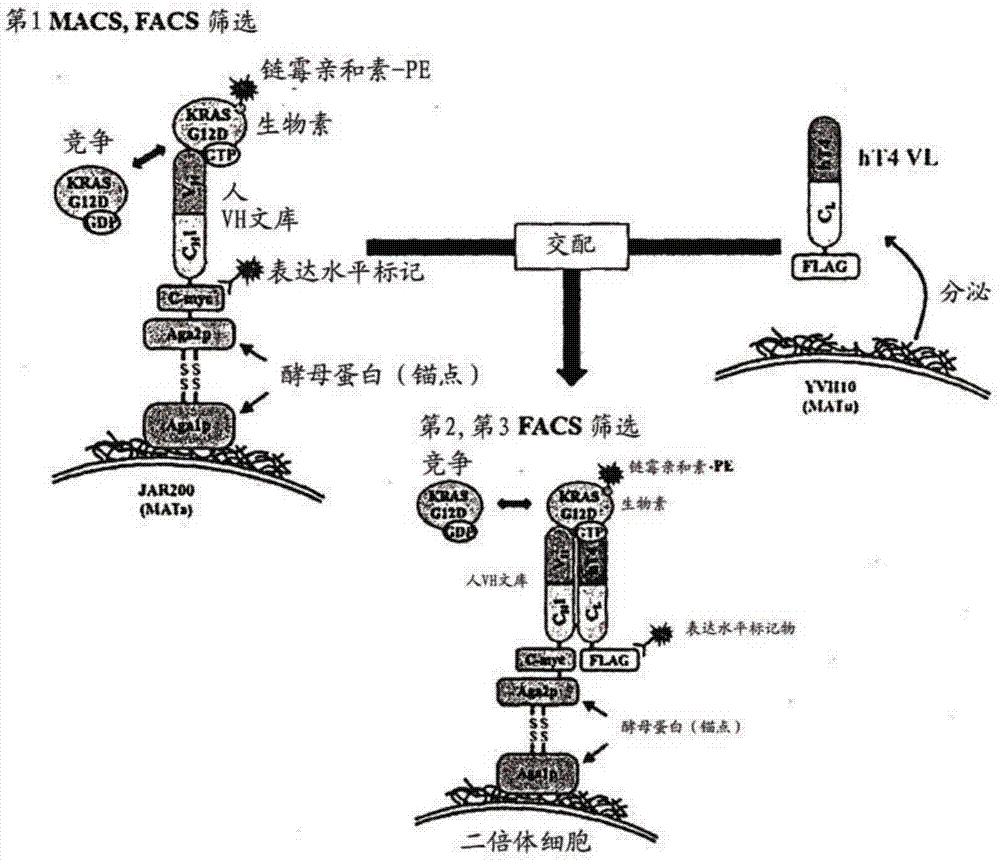
[0210] Figure 15 A schematic diagram showing the library screening strategy to obtain humanized antibody heavy chain variable single domains with high affinity only for the GTP-bound KRas G12D protein.
[0211] Specifically, the GTP-bound KRas G12D purified in Example 14 was biotinylated (EZ-LINK TM Sulfo-NHS-LC-Biotinylation Kit (Pierce Inc., USA)) and then reacted with the heavy chain variable region library displayed on the yeast cell surface for 1 hour at room temperature. A library of heavy chain variable regions reacted with biotinylated GTP-conjugated KRas G12D on the surface of yeast cells was reacted with streptavidin (Microbead TM (Miltenyi Biotec) was reacted at 4°C for 20 minutes and then MACS (Magnetic Activated Cell Sorting) was used to enrich yeasts exhibiting heavy chain variable regions with high affinity for GTP-KRAS G12D. The selected library display ye...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com