Preparation method of cyclic propyl phosphonic anhydride
A technology of propylphosphonic anhydride and propylphosphonic acid, which is applied in the field of preparation of cyclic propylphosphonic anhydride, can solve the problems that cannot meet the needs of lithium battery additives, is not suitable for industrial production, and the reaction process is easy to get out of control, and achieves low cost , the preparation method is simple, the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A preparation method of cyclic propylphosphonic anhydride, the steps are as follows:
[0042] (1) Preparation of propylpyrophosphonic acid: Add 89.0g (720mmol) propylphosphonic acid, 38.8g (380mmol) acetic anhydride and 200mL xylene into a 500mL three-necked flask, fully replace with nitrogen, heat to 128°C, and react for 7.0 h; after completion of the reaction, a vacuum distillation device was installed to evaporate the reaction solvent xylene and by-product acetic acid to obtain 80.2 g of viscous liquid propyl pyrophosphonic acid, with a yield of 96.80%;
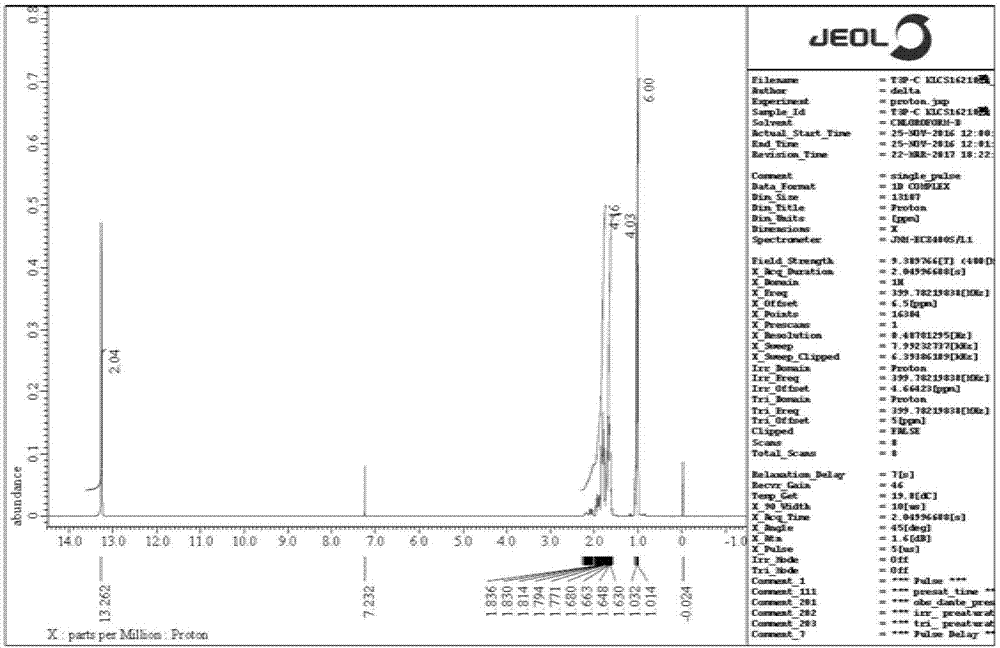
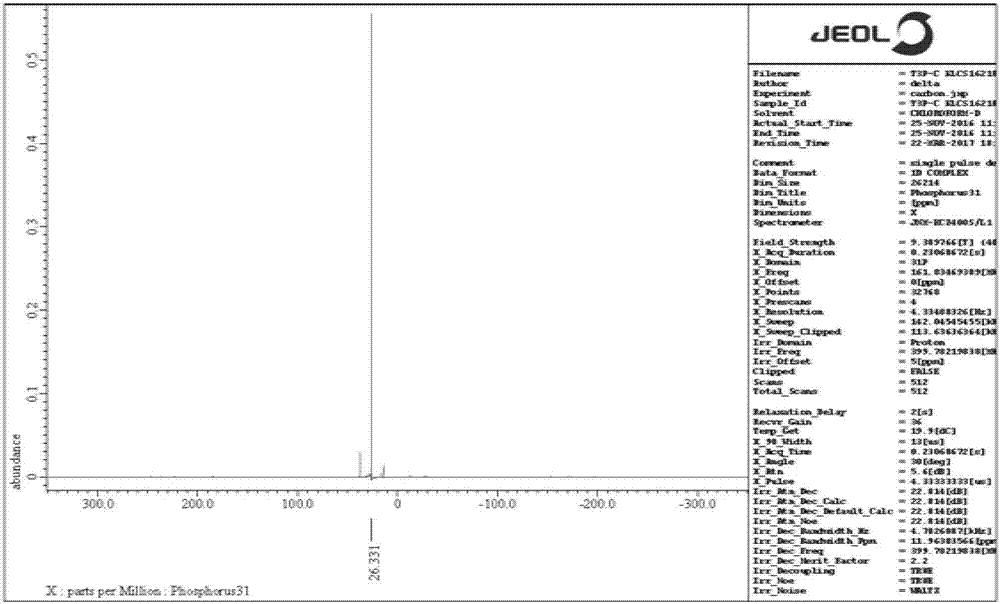
[0043] 1 HNMR (400MHz, CDCl 3 ), δ (ppm): 13.262 (s, 2H), 1.836-1.630 (m, 8H) and 1.065-0.996 (m, 6H); 31 PNMR (161.8MHz, CDCl 3 ), δ(ppm): 26.063;
[0044] (2) Preparation of propylphosphonochloride: Add 89.0g (720mmol) propylphosphonic acid, 188.5g (1584mmol) thionyl chloride and 300mL toluene into a 1L three-necked flask, fully replace with nitrogen, heat to 80°C, and react for 4.0 h; After the reaction is c...
Embodiment 2
[0050] A preparation method of cyclic propylphosphonic anhydride, the steps are as follows:
[0051] (1) Preparation of propylpyrophosphonic acid: Add 89.0g (720mmol) propylphosphonic acid, 39.8g (390mmol) acetic anhydride and 200mL toluene into a 500mL three-necked flask, fully replace with nitrogen, heat to 105°C, and react for 8.0h After completion of the reaction, a vacuum distillation device was installed to evaporate the reaction solvent toluene and by-product acetic acid to obtain 79.9 g of viscous liquid propyl pyrophosphonic acid, with a yield of 96.38%;
[0052] 1 HNMR (400MHz, CDCl 3 ), δ (ppm): 13.262 (s, 2H), 1.836-1.630 (m, 8H) and 1.032-0.996 (m, 6H); 31 PNMR (161.8MHz, CDCl 3 ), δ(ppm): 26.331;
[0053] (2) Preparation of propylphosphonochloride: Add 89.0g (720mmol) propylphosphonic acid, 176g (1476mmol) thionyl chloride and 300mL toluene into a 1L three-necked flask, fully replace with nitrogen, heat to 70°C, and react for 6.0h After the completion of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com