Drug for treating malignant tumors
A malignant tumor and drug technology, applied in the direction of antineoplastic drugs, drug combinations, antibacterial drugs, etc., can solve the problems of limited application and achieve the effect of enriching the library of therapeutic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
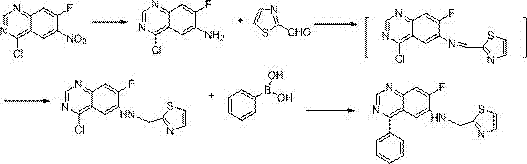
[0034] Example 1: Synthesis of 2-thiazolylmethyl-4-phenyl-7-fluoro-quinoxalinyl-6-secondary amine
[0035] The synthetic route is:
[0036]
[0037] Synthetic steps:
[0038] 1-1 Synthesis of 4-chloro-7-fluoro-quinazolinyl-6-amine
[0039]
[0040] Add 40 ml of methanol and tetrahydrofuran (3:1 by volume) to 4-chloro-7-fluoro-6-nitro-quinazoline (10 mmol), stir for 20 minutes, dissolve completely, and then cool down to 0-5°C, add 1 g of sodium borohydride to it, keep the temperature and stir for two hours, then rise to room temperature and continue to stir for 4 hours, add 40 ml of water to the reaction, extract with 50 ml of ethyl acetate, and use anhydrous Dry over sodium sulfate for 2 hours, filter, evaporate the organic solvent to dryness, and dry under vacuum at 50°C to obtain 1.8 g of a yellow solid, which is 4-chloro-7-fluoro-quinazolinyl-6-amine, with a yield of 91%. 1 H-NMR (400 MHz, CDCl 3 ) δ: 6.94(m,2H), 7.04(s,2H), 9.56(s,1H). 13 C-NMR (75 MHz, CDCl 3 ...
Embodiment 2
[0050] Example 2: Synthesis of 2-thiazolylmethyl-4-(2-naphthyl)-7-fluoro-quinoxalinyl-6-secondary amine
[0051]
[0052] The synthesis method is as 1-4 in Example 1, and 2-thiazolylmethyl-4-chloro-7-fluoro-quinoxalinyl-6-secondary amine (10 mmol) is dissolved in 30 ml of nitrogen-nitrogen dimethylformamide During the process, the system was blown with argon for 20 minutes, and the air in the system was evacuated, then tetrakistriphenylphosphine palladium was added, the temperature was raised to 60°C, and stirring was continued for half an hour, 2-naphthylboronic acid (12 mmol) was added thereto, and then 10 milliliters of sodium carbonate (1 gram) aqueous solution was warmed up to 90° C., stirred for 5 hours, kept feeding argon during the whole process, then lowered the temperature, evaporated the solvent under reduced pressure, and the solid passed through the chromatographic column quickly.
[0053] 3.4 g of off-white 2-thiazolylmethyl-4-(2-naphtenyl)-7-fluoro-quinoxalin...
Embodiment 3
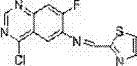
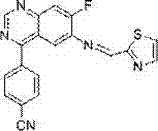
[0054] Example 3: Synthesis of 4-[7-fluoro-6-(2-thiazolylmethylamino)]quinoxalinyl-benzonitrile
[0055]
[0056] The synthesis method is as 1-4 in Example 1, and 2-thiazolylmethyl-4-chloro-7-fluoro-quinoxalinyl-6-secondary amine (10 mmol) is dissolved in 30 ml of nitrogen-nitrogen dimethylformamide In the system, the system was blown with argon for 20 minutes, and the air in the system was evacuated, then tetrakistriphenylphosphine palladium was added, the temperature was raised to 60°C, and stirring was continued for half an hour, p-cyanophenylboronic acid (12 mmol) was added thereto, and then Add 10 milliliters of sodium carbonate (1 gram) aqueous solution, raise the temperature to 90° C., stir for 5 hours, keep feeding argon during the whole process, then lower the temperature, evaporate the solvent under reduced pressure, and the solid quickly passes through the chromatographic column to obtain 3.3 grams of light yellow 4- [7-Fluoro-6-(2-thiazolylmethylamino)]quinoxali...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com