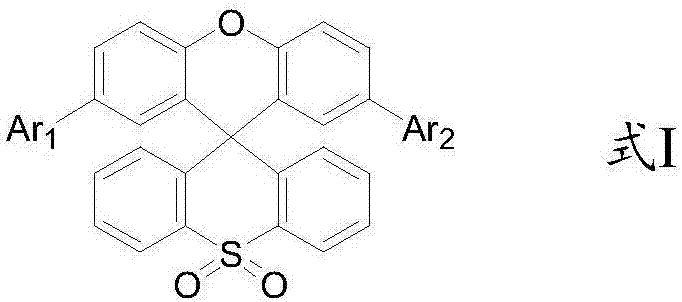
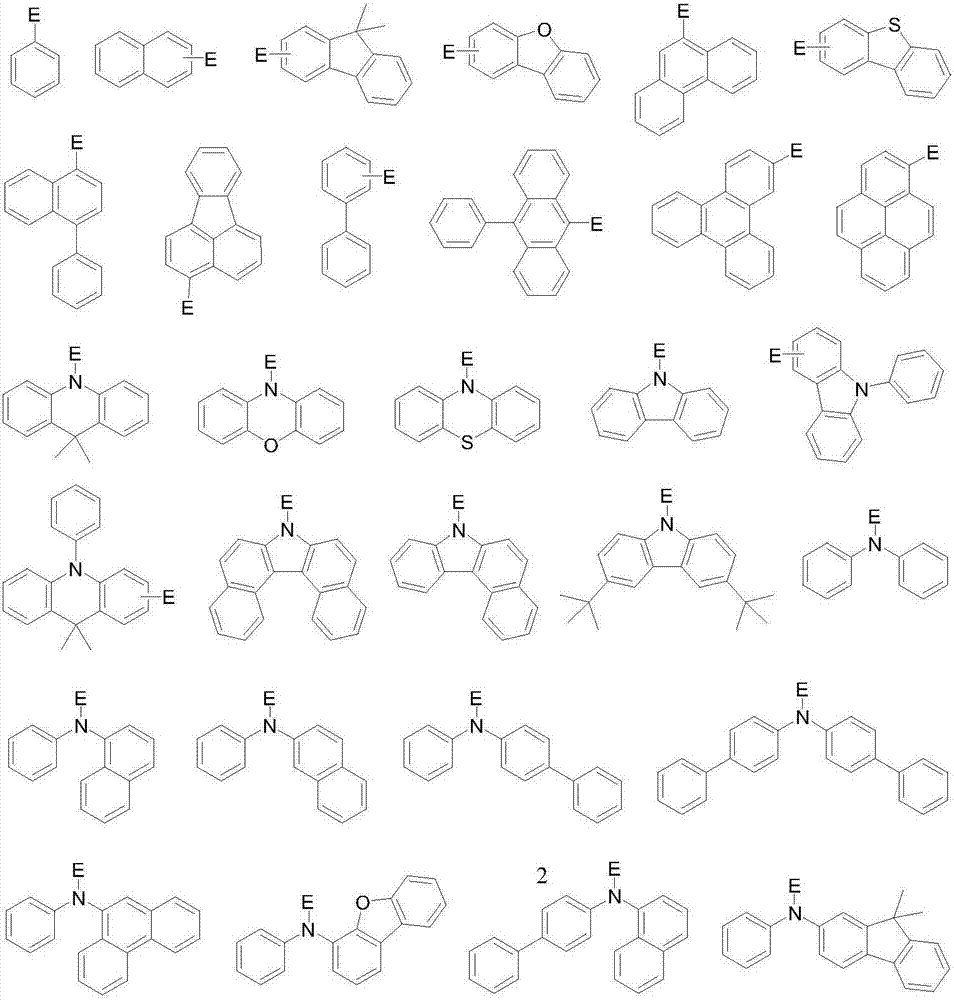
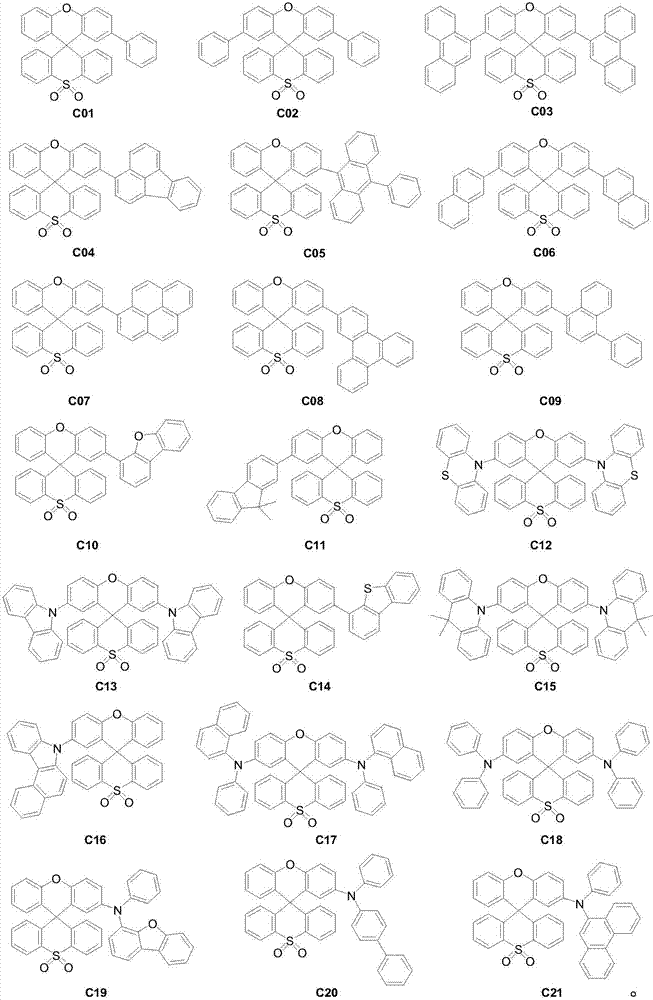
Organic light-emitting material with xanthene spirothioxanthene oxide as parent nucleus and application thereof
An electroluminescent material, spirothiaxanthene technology, applied in the direction of luminescent materials, organic chemistry, circuits, etc., can solve the problems of increasing the difficulty of high-efficiency TADF main materials, quenching, low device performance, etc., and achieve good industrialization prospects , enhanced stability, and good photoelectric performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of compound C03
[0031]
[0032] Under nitrogen protection, raw material A-1 (1.62g, 2.5mmol), raw material B-1 (1.41g, 5.5mmol), 80mL toluene and 20mL water were added to a 250mL three-necked flask, and then the catalyst tetrakis(triphenylphosphine ) palladium (0.029g, 0.025mmol), acid-binding agent potassium carbonate (1.04g, 7.5mmol). The temperature of the system was raised to reflux for 8 hours, and the temperature was naturally lowered to 20-25° C., the liquid was separated, the solvent was removed, and the crude product was crystallized with toluene to obtain 1.62 g of target C03 with a yield of 86.6%.
[0033] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 53 h 32 o 3 S, theoretical value 748.207, test value 748.206. Elemental analysis (C 53 h 32 o 3 S), theoretical value C: 85.00, H: 4.31, O: 6.41, S: 4.28, measured value C: 80.01, H: 4.30, O: 6.40, S: 4.29.
Embodiment 2
[0034] Embodiment 2: the preparation of compound C10
[0035]
[0036] The synthesis method refers to the preparation method of C03, and the total yield is 76.8%.
[0037] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 37 h 22 o 4 S, theoretical value 562.124, test value 562.123. Elemental analysis (C 37 h 22 o 4 S), theoretical value C: 78.99, H: 3.94, O: 11.37, S: 5.70, measured value C: 78.10, H: 3.93, O: 11.37, S: 5.70.
Embodiment 3
[0038] Embodiment 3: the preparation of compound C11
[0039]
[0040] The synthesis method refers to the preparation method of C03, and the total yield is 77.1%.
[0041] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 40 h 28 o 3 S, theoretical value 588.176, test value 588.177. Elemental analysis (C 40 h 28 o 3 S), theoretical value C: 81.61, H: 4.79, O: 8.15, S: 5.45, measured value C: 81.61, H: 4.79, O: 8.15, S: 5.45.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum current efficiency | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com