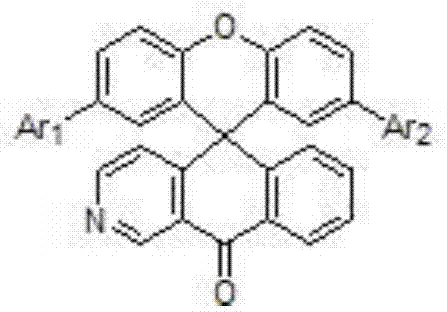
Organic electroluminescent material with xanthene spiroazaanthrones, preparation method and application thereof
A technology of xanthene spiro azaxanthone and xanthene spiro azaxanthone is applied in the field of organic electroluminescence, which can solve the requirement and market demand that cannot meet the performance improvement of OLED devices, and increase the difficulty of high-efficiency TADF host materials , low device performance, etc., to achieve good industrialization prospects, inhibit quenching effect, and good photoelectric performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
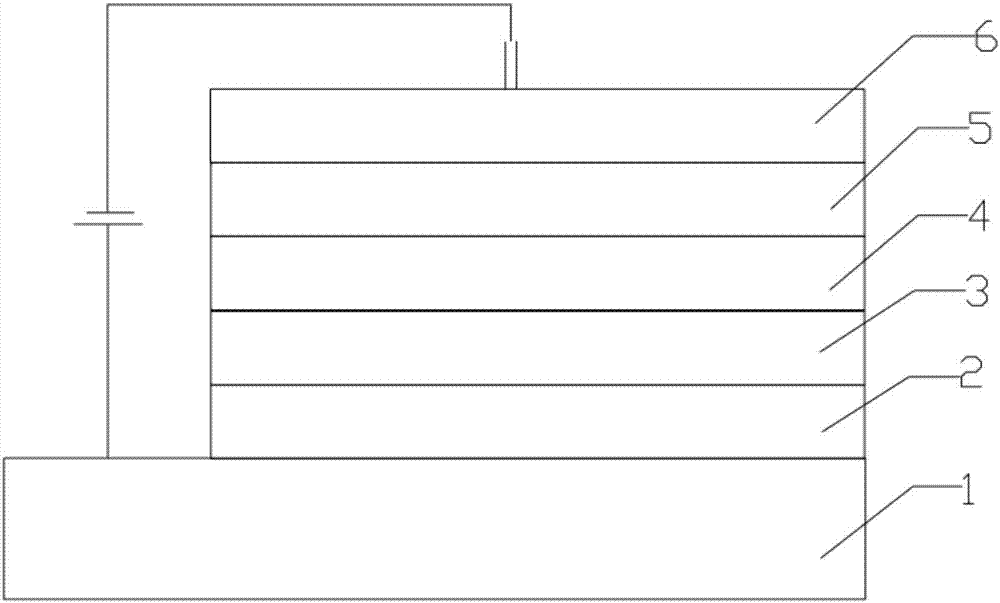
Image
Examples
Embodiment 1
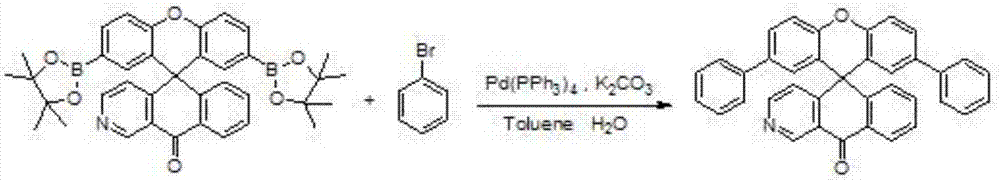
[0032] The synthesis of embodiment 1 compound C01
[0033]
[0034] Under nitrogen protection, raw material II (1.53g, 2.5mmol), bromobenzene (0.86g, 5.5mmol), 80mL toluene and 20mL water were added to a 250mL three-necked flask, and then the catalyst tetrakis(triphenylphosphine) palladium (0.029 g, 0.025mmol), acid-binding agent potassium carbonate (1.04g, 7.5mmol). The temperature of the system was raised to reflux for 8 hours, the temperature was naturally lowered to 20-25° C., the liquid was separated, the solvent was removed, and the crude product was crystallized with toluene to obtain 1.12 g of the target product C01 with a yield of 87.5%.
[0035] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 37 h 23 NO 2 , the theoretical value is 513.173, and the test value is 513.173. Elemental analysis (C 37 h 23 NO 2 ), theoretical values C: 86.53, H: 4.51, N: 2.73, O: 6.23. Found values C: 86.53, H: 4.50, N: 2.74, O: 6.23.
Embodiment 2
[0036] The synthesis of embodiment 2 compound C05
[0037]
[0038] Referring to Example 1, the target product C05 was obtained with a yield of 76.6%.
[0039] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 43 h 26 N 2 o 2 , the theoretical value is 602.199, and the test value is 602.199. Elemental analysis (C 43 h 26 N 2 o 2 ), theoretical values C: 85.69, H: 4.35, N: 4.65, O: 5.31. Found values C: 85.70, H: 4.34, N: 4.65, O: 5.31.
Embodiment 3
[0040] The synthesis of embodiment 3 compound C08
[0041]
[0042] Referring to Example 1, the target product C08 was obtained with a yield of 76.1%.
[0043] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 41 h 25 NO 2 , the theoretical value is 563.189, and the test value is 563.181. Elemental analysis (C 41 h 25 NO 2 ), theoretical values C: 87.37, H: 4.47, N: 2.49, O: ,5.68. Found values C: 87.37, H: 4.47, N: 2.49, O: 5.68.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com