Targeted fusion protein against HBV replication and construction method thereof
A technology of fusion protein and construction method, applied in the field of targeted fusion protein, can solve the problems of side effects, treatment failure, easy to produce tolerance and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0049] The present invention will be described in detail below in combination with specific embodiments.
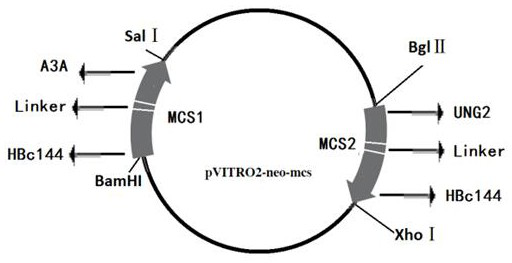
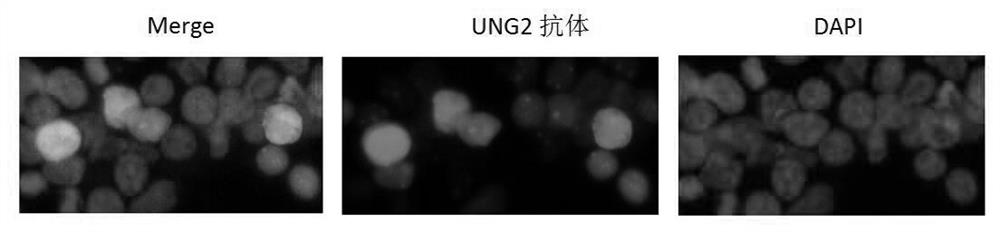
[0050] In order to enhance the effect of A3A and UNG2 on degrading cccDNA, the present invention localizes A3A and UNG2 to the nucleus and specifically binds to the characteristics of cccDNA, and uses HBV core protein (HBc) as the targeting molecule of A3A and UNG2 to guide A3A and UNG2 into the nucleus and interact with it. Combined with cccDNA, play the role of degrading cccDNA. HBc is a structural protein that assembles the viral nucleocapsid, and has the characteristic of specifically binding to HBV cccDNA, and the region that determines this characteristic is the C-terminal region (CTD) of HBc, and this region also has the function of nuclear localization.
[0051] HBc is the core protein of HBV, which is a structural protein for assembling the viral nucleocapsid. HBc is 183aa long and is divided into two parts: N-terminal region (1-143aa) and C-terminal region (CTD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com