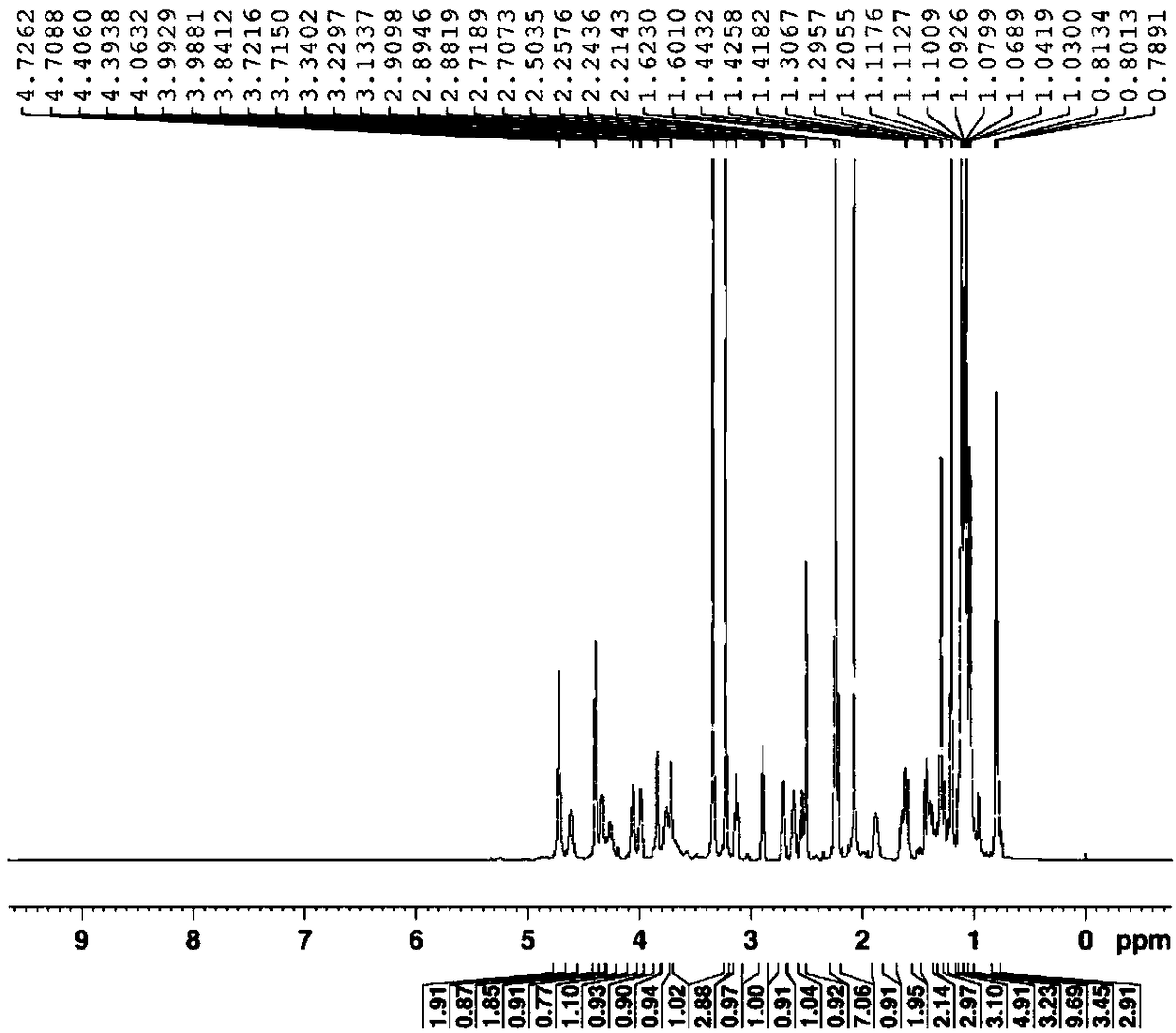
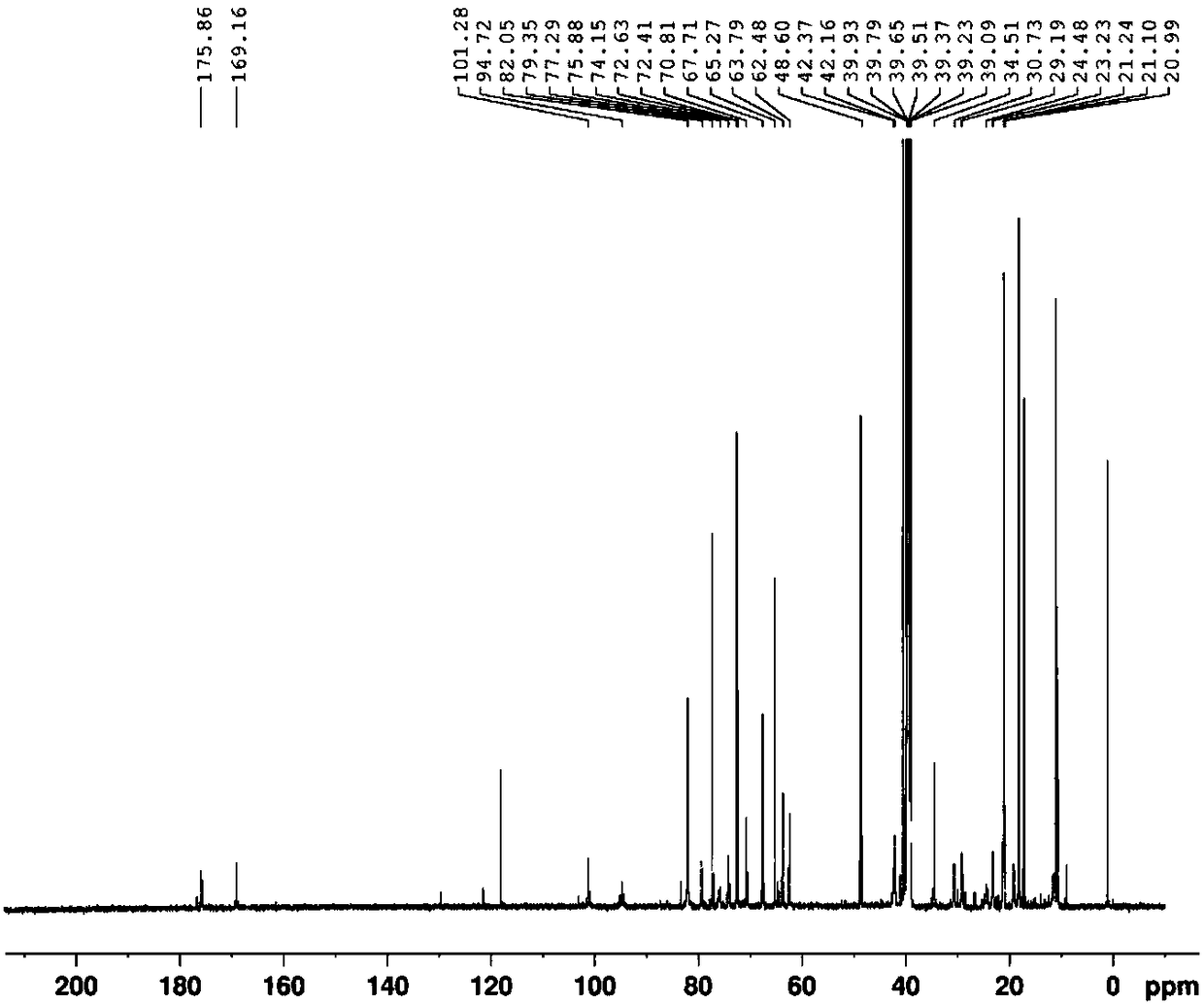
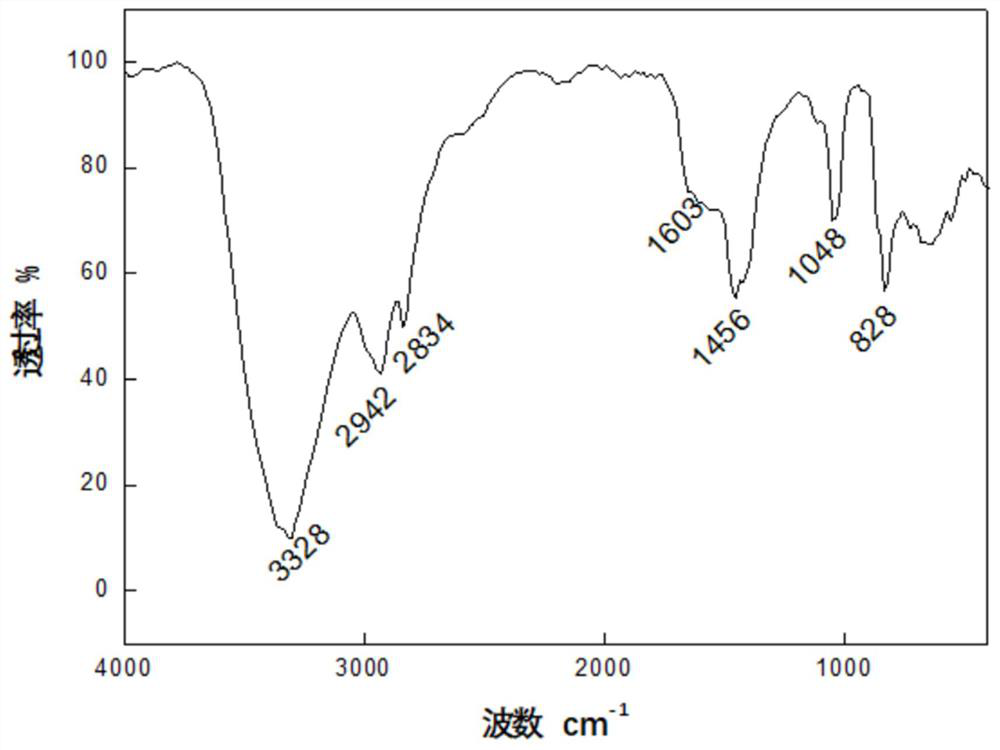
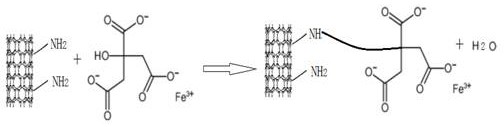
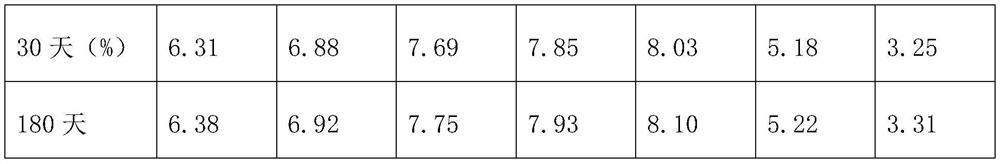
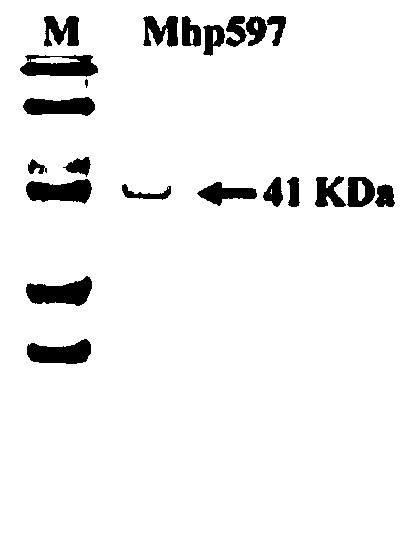Patents
Literature
35results about How to "Promote degradation reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Multi-degradable plastic film and preparation method thereof
The invention discloses a multi-degradable plastic film which comprises the following components in percentage by weight: 85-98.7% of a polymer substrate, 0.1-1% of nano-titanium dioxide, 0.1-1% of kaolin, 0.1-1% of ferric stearate and 1-2% of hydrophilic resin. A preparation method of the multi-degradable plastic film comprises the following steps: uniformly mixing polyolefin, nano-titanium dioxide, kaolin, ferric stearate and hydrophilic resin in percentage by mass, melting and pelleting, and performing blow molding by using a blow molding machine, thereby preparing the multi-degradable plastic film. The multi-degradable plastic film has the beneficial effects that firstly, the use property of a product is not affected, and the basic requirements of daily life, agriculture use and the like can be met; secondly, multi-degradation can be achieved by using light, heat and biological substances, and due to the added hydrophilic resin, the plastic film can easily absorb the moisture in the air and the soil, multi-degradation is accelerated, and complete degradation is achieved.
Owner:TIANJIN LISHUN PLASTIC PRODS
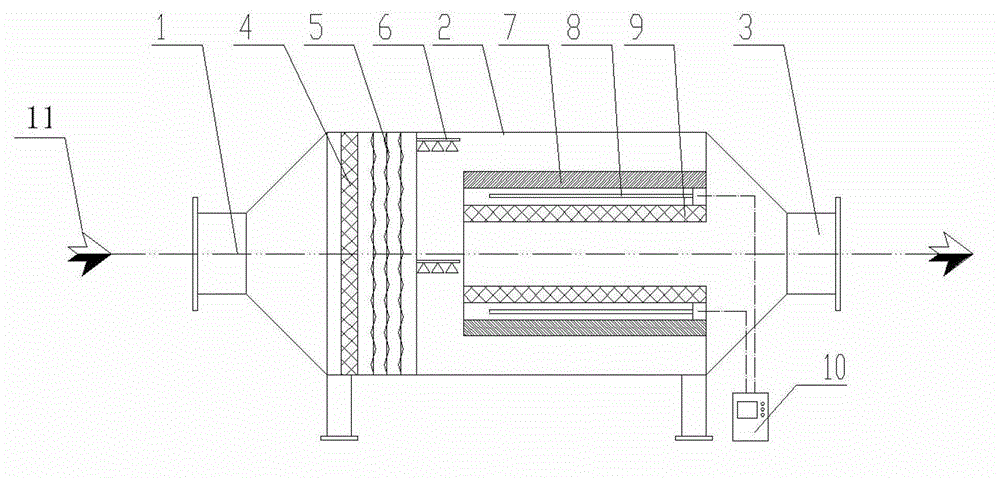
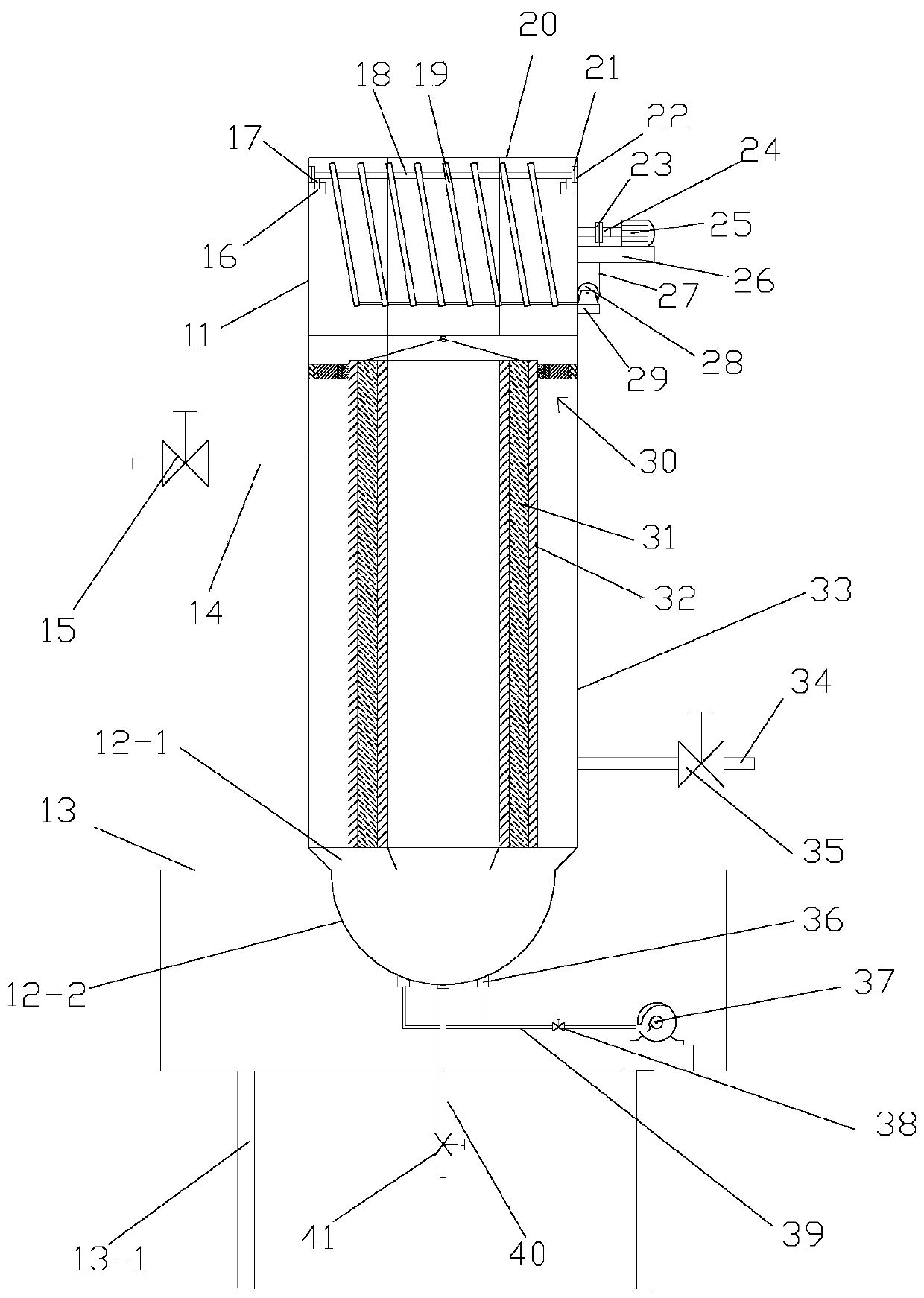
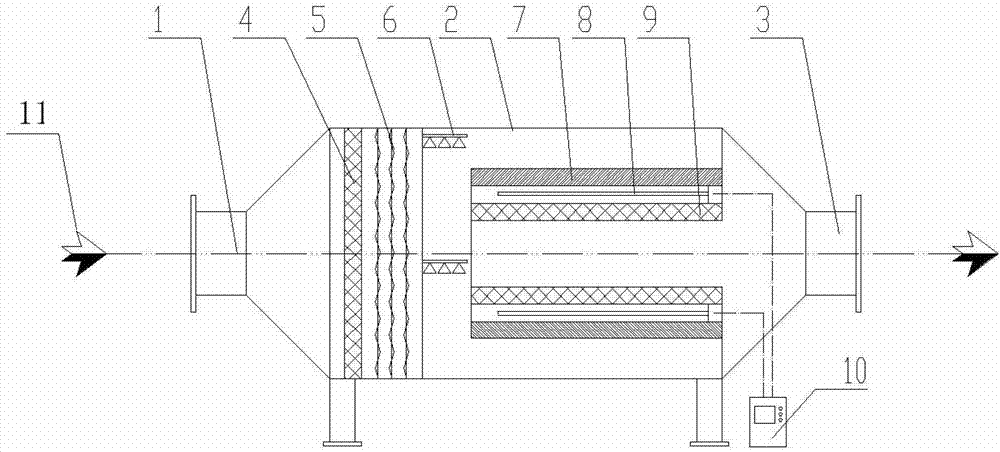
Device for treating organic waste gas by applying photochemical technology
ActiveCN102861504AEfficient decompositionPromote degradation reactionDispersed particle filtrationPlasma reactorResource consumption
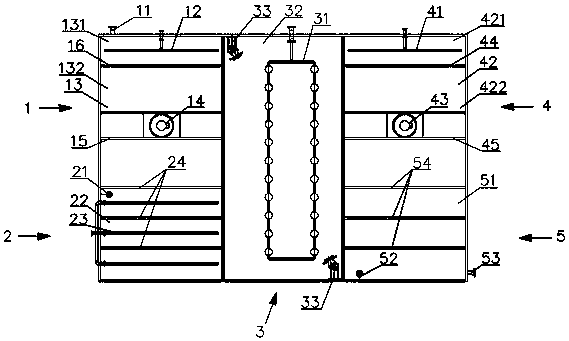
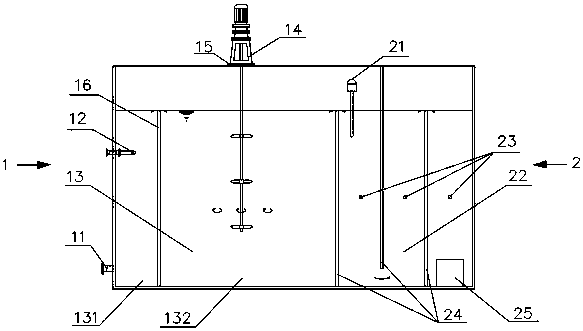
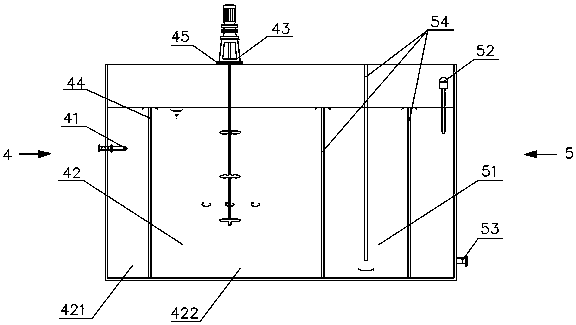
A device for treating organic waste gas by applying a photochemical technology comprises a high-strength security filter screen, a plasma reactor, a liquid mist humidifier, a catalytic reaction screen, an adsorption reaction screen, an ultraviolet lamp unit and an electric controller; the catalytic reaction screen and the adsorption reaction screen are subjected to the irradiation of the ultraviolet lamp unit; the organic waste gas is purified after being filtered by the high-strength filter screen, decomposed by the plasma reactor, humidified and cooled by the liquid mist humidifier, catalyzed by the catalytic reaction screen and adsorbed by the adsorption reaction screen; and the electric controller is connected to the plasma reactor and the ultraviolet lamp unit and used for controlling the starting and stopping of the plasma reactor and the ultraviolet lamp unit. The device for treating the organic waste gas by applying the photochemical technology can be used for efficiently treating industrial organic waste gas, does not produce secondary pollution residues and is lower in resource consumption and operation cost.
Owner:GUANGDONG SENYANG ENVIRONMENT PROTECTION ENG & FACILITIES
Method for degrading organic pollutants based on biochar
InactiveCN111906138ANot affected by pHPromote degradation reactionWater treatment compoundsWater contaminantsSulfate radicalsSuperoxide
The invention discloses a method for degrading organic pollutants based on biochar, which comprises the following steps: regulating the pH value of the organic pollutants to 3-9, adding ferric iron salt, persulfate and biochar, stirring the components, and carrying out oxidative degradation treatment at room temperature for 0.5-5 hour. According to the method, the oxidation-reduction capacity of the charcoal is fully utilized, wherein electrons are provided for reducing variable-valence metal so as to activate persulfate, sulfate radicals, hydroxyl and superoxide anion free radicals are generated, and therefore efficient degradation of pollutants is achieved. The method has a good application prospect in in-situ chemical oxidation remediation of actual organic matter contaminated soil andunderground water.
Owner:KUNMING UNIV OF SCI & TECH
Organic wastewater catalytic degradation and separation device and method
PendingCN110330073ASimple structureReasonable designWater/sewage treatment by irradiationWater treatment compoundsWastewaterTitanium dioxide
The invention discloses an organic wastewater catalytic degradation and separation device and method. The device comprises a dilution mechanism, a catalytic degradation and separation mechanism, a first control module and a second control module. The diluting mechanism comprises a diluting box, a stirring mechanism and a stirring driving mechanism. The catalytic degradation separation mechanism comprises a base bracket, a separation chamber, a catalytic mechanism and an aeration mechanism. The separation chamber comprises an upper separation chamber and a middle catalytic degradation chamber and a recovery chamber, wherein a titanium dioxide particle catalyst is arranged in the recovery chamber, and a separation mechanism is arranged in the upper separation chamber. The method comprises the following steps: 1, diluting organic wastewater; 2, conveying the diluted organic wastewater; 3, adding a titanium dioxide particle catalyst; and 4, carrying out catalytic degradation and separationon the diluted organic wastewater. According to the invention, dilution of organic wastewater is realized, catalytic degradation is carried out on the diluted organic wastewater, the catalytic degradation efficiency is improved, and the titanium dioxide particle catalyst can be separated and recovered.
Owner:SHAANXI NORMAL UNIV
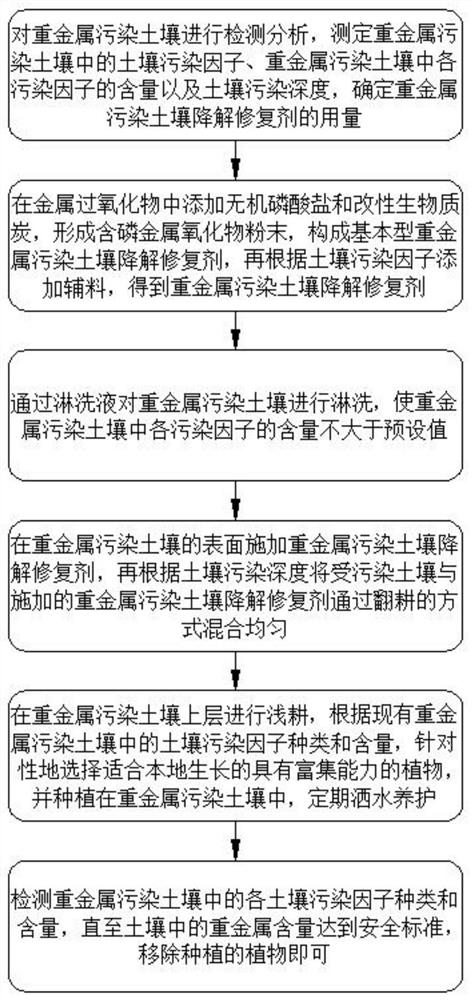
Heavy metal contaminated soil degradation harmless treatment method
InactiveCN113058985ANo secondary pollutionAvoid secondary pollutionContaminated soil reclamationSoil scienceEngineering
The invention discloses a heavy metal contaminated soil degradation harmless treatment method which comprises the following steps: S1, detecting and analyzing heavy metal contaminated soil, and determining the dosage of a heavy metal contaminated soil degradation repairing agent; S2, preparing the heavy metal contaminated soil degradation repairing agent; S3, leaching; S4, applying the heavy metal contaminated soil degradation repairing agent, and performing ploughing and mixing; S5, planting plants which are suitable for local growth and have enrichment capacity; and S6, detecting until the heavy metal content in the soil reaches the safety standard. Harmless treatment of the heavy metal contaminated soil is achieved, and the requirements of resource utilization, soil replacement and landfill are met; the technology application range is wide, and the method is suitable for remediation of various soils contaminated byheavy metals,including chromium, hexavalent chromium, lead, zinc, copper, chromium, nickel, mercury, arsenic and the like; the technical operation is simple and convenient, the repairing time is short, the capacity increase is less, secondary pollution is avoided, and the cost is lower; and the resource utilization of the polluted soil can be realized, and the repaired soil is suitable for various purposes.
Owner:益壤(厦门)环保科技有限公司
Method for degrading chitosan through microwave-enzyme method coupling
The invention belongs to the technical field of chitosan preparation and relates to a method for degrading chitosan through microwave-enzyme method coupling. The method comprises the steps as follows: firstly, dissolving the high-molecular-weight chitosan in an acetic acid-sodium acetate buffer solution with the pH value in a range of 4-6, and fully mixing the chitosan and the buffer solution to prepare a chitosan solution; then adding an enzyme with the mass accounting for 0.5-3% of the mass of the chitosan into the prepared chitosan solution, pouring the chitosan solution into a reaction bottle after even mixing, then placing the reaction bottle into a microwave reactor, feeding a nitrogen gas into the reaction bottle, and controlling the temperature to be below 65 DEG C and the microwave power in a range of 100-1000 W to have a degradation reaction for 10-180 min; after the degradation reaction is finished, heating a microwave to 100 DEG C to inactivate the enzyme for 10-15 min; finally, adjusting the pH value of the reacted solution to be in a range of 7-8 with an alkaline solution, removing sediment through filtration, removing saline ions from an obtained filtrate with a dialysis method, and then freezing and drying the filtrate to prepare the low-molecular-weight chitosan. The method is simple to operate, easy to control, mild in condition and environment-friendly.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
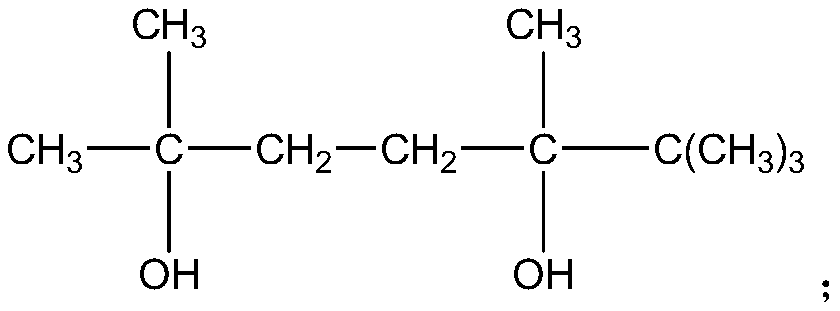
Wool-like polyester filament and preparation method thereof
ActiveCN109750372AIncrease the free volume of spaceIncrease the void free volumeMonocomponent copolyesters artificial filamentMonocomponent polyesters artificial filamentPolymer scienceNatural degradation
The invention relates to a wool-like polyester filament and a preparation method thereof. The preparation method comprises the steps: firstly, terephthalic acid, 1,4-butanediol, fluorinated diacid, hexanediol with tertiary butyl side groups, and 2,5,6,6-tetramethyl-2,5-heptanediol are evenly mixed, and then an esterification reaction and a polycondensation reaction are carried out successively toprepare modified polyester; then a modified polyester POY filament is prepared from modified polyester flux according to a POY process; and finally a modified polyester DTY filament is prepared from the modified polyester POY filament according to a DTY process, and then the wool-like polyester filament is obtained. According to a prepared product, when the temperature is 100 DEG C, the dye-uptakeis 90.32-93.27%, and the K / S value is 22.15-23.42; after the product is placed at temperature of 25 DEG C and the relative humidity of 65% for 60 months, the intrinsic viscosity of the product is lowered by 17-20%; and the preparation method is low in cost and simple in process, and the prepared product is excellent in dyeing property and high in natural degradation speed, and has great application prospects.
Owner:JIANGSU HENGLI CHEM FIBER
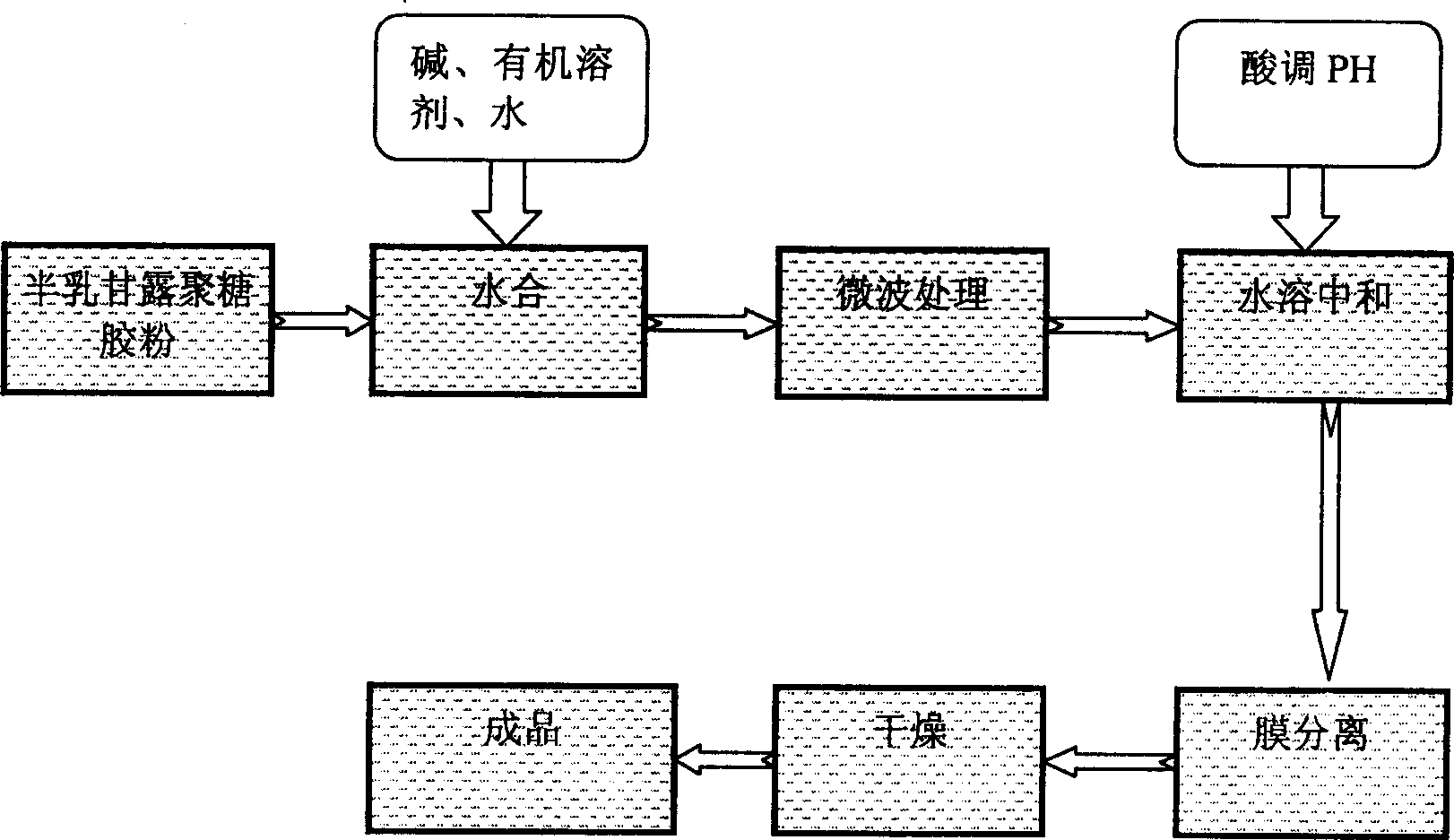
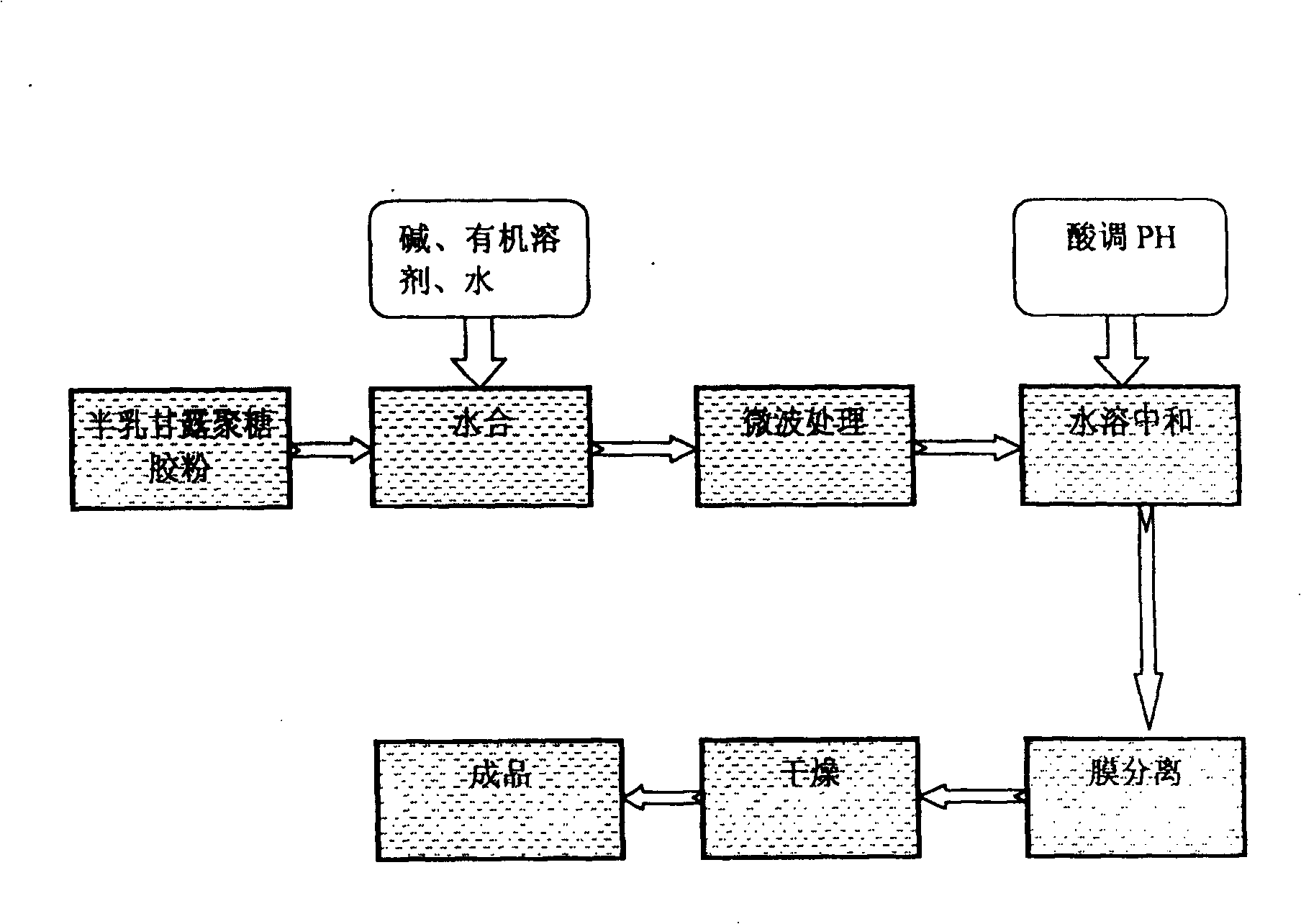
Semi-dry microwave treating process of preparing galactomannan
The present invention discloses semi-dry microwave treating process of preparing galactomanoligose. Galactomanoligose is prepared with galactomannan powder as material and through the steps of hydration, microwave treatment, water dissolving and neutralizing, membrane separating and drying. The process has short production period, high production efficiency, less pollution and low power consumption.
Owner:昆明旭日丰华农业科技有限公司
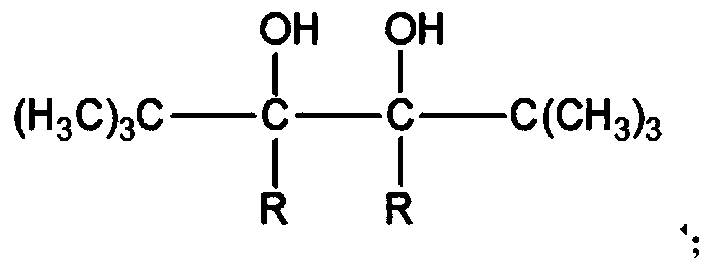
Flame-retardant polyester fiber and preparation method thereof
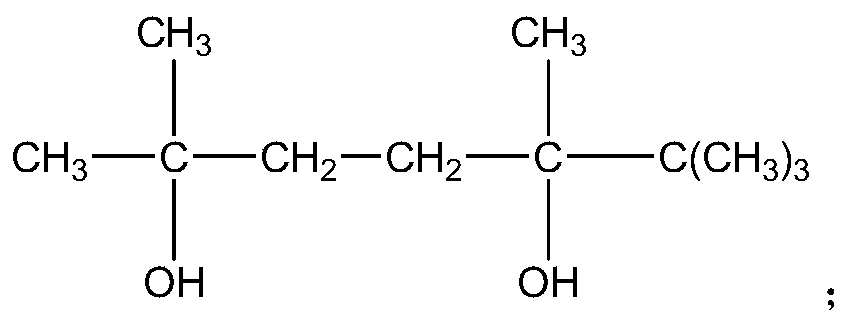
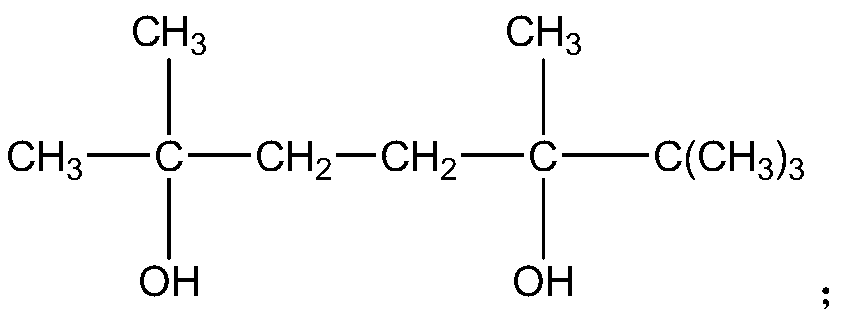
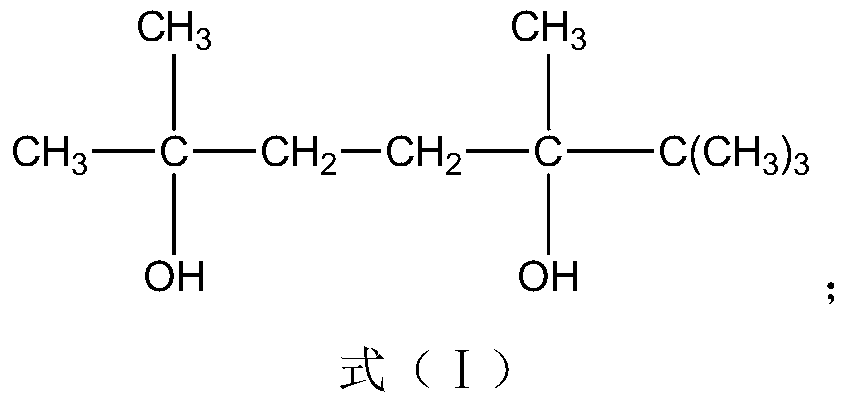
ActiveCN109706542AIncrease the free volume of spaceIncrease the void free volumeMonocomponent polyesters artificial filamentArtifical filament manufactureFiberYarn
The invention relates to a flame-retardant polyester fiber and a preparation method thereof. The preparation method includes the step that modified polyester FDY yarn, namely the flame-retardant polyester fiber, is prepared from a modified polyester melt according to the FDY process. A preparation method of modified polyester comprises the step that terephthalic acid, ethylene glycol, 2-CEPPA, 2,5,6,6-tetramethyl-2,5-heptandiol and doped modified Bi2O3 powder are evenly mixed and subjected to an esterification reaction and a polycondensation reaction in sequence, wherein the structural formulaof 2,5,6,6-tetramethyl-2,5-heptandiol is shown in the description. The dye-uptake of the prepared product is 85.3-89.2% under the temperature condition of 120 DEG C, and the K / S value is 22.35-25.43;after the product is placed for 60 months under the condition that the temperature is 25 DEG C and the relative humidity is 65%, the characteristic viscosity of the product is reduced by 13-18%. Thepreparation method is low in cost and simple in process; the prepared product has excellent dyeing property and natural degradability.
Owner:JIANGSU HENGLI CHEM FIBER
Method for preparing chitosan oligosaccharides through semi-wet method microwave treatment
A method for preparing chitosan oligosaccharides through semi-wet method microwave treatment relates to a production method of chitosan oligosaccharides. The method comprises the following steps: using chitosan gelatine powder as raw material to perform hydration and microwave treatment, dissolving in water, neutralizing, and performing membrane separation, drying and the like to prepare chitosan oligosaccharides. The method does not consume organic solvent, thus the problem of recycling solvent does not exist and the pollution is low; and as the volume of the reaction system is from 1 / 3 to 1 / 5 of that of the solution method, the production efficiency is obivously increased. The method adopts microwave treatment to promote the polysaccharide degradation reaction, thus the heating effect is greatly increased. The radiation thermal effect of microwave and the non-thermal effect of microwave are utilized to prepare the product. Meanwhile, microwave is an efficient and clean energy, thus the speed of the chemical reaction can be greatly increased. The method also adopts the membrane separation mean, thus the refining efficiency is high and the product quality is controllable.
Owner:YANGZHOU RIXING BIO TECH +1
Preparation method of window screen coating for air purification
InactiveCN108329809AGet rid of hard-to-clean annoyancesQuick breakdownAntifouling/underwater paintsPaints with biocidesParticulatesTitanium oxide
The invention relates to the technical field of window screens, in particular to a preparation method of a window screen coating for air purification. The preparation method comprises steps as follows: (1) materials are prepared; (2) 3-ethyl-3-oxetanemethanol is dissolved in an organic solvent, an initiator, a titanium source, a zinc source and kaolin are added, the mixture is subjected to refluxat 50-60 DEG C for 5-10 h, a precipitator is added for precipitation, a precipitate is dried to the constant weight under the vacuum condition, the temperature is increased to 270-300 DEG C, a productis continuously calcined for 1-3 h, and modified hyperbranched polyether is obtained; (3) the modified hyperbranched polyether in the step (2) and colloidal graphite powder are mixed and dispersed insolvent type thermosetting resin, a diluent and a curing agent are matched, the mixture is stirred at a high speed for 15-20 min, and the window screen coating is obtained. The titanium source and the zinc source are introduced into the forming process of hyperbranched polyether and are converted into titanium oxide and zinc oxide in situ in molecular pore channels of the hyperbranched polyether,organic matters in adsorbed particulate pollutants are rapidly degraded, and air is rapidly purified.
Owner:合肥宸翊商贸有限公司
Intelligent biomass straw degradation device and use method thereof
InactiveCN107435024AIncrease temperatureAccelerate the rate of degradation reactionBioreactor/fermenter combinationsBiological substance pretreatmentsCelluloseAutomatic control
The invention relates to an intelligent biomass straw degradation device and a using method thereof, and belongs to the technical field of preparation of biological feeds. The device comprises a rack and a sealed degradation tank; and the top of the sealed degradation tank is provided with a feed inlet, the bottom of the sealed degradation tank is provided with a discharge opening, the wall of the tank is provided with a display controller, the center in the tank is provided with a stirring shaft, a pressure sensor and a temperature sensor are arranged on the inner wall of the tank, a helical heating tube is wound around the outer side of the sealed degradation tank, an ozone generator is arranged at one side of the degradation tank, the outlet of the ozone generator communicates the internal of the sealed degradation tank through an ozone conveying tube, and the display controller automatically controls the on-off of the spiral heating tube and the closing and opening of a flow control valve in order to maintain the optimal reaction conditions in the sealed degradation tank. Compared with traditional degradation devices, the device in the invention has the advantages of realization of uniform and sufficient contact of biomass straws with ozone, increase of the temperature in the sealed degradation tank, facilitation of the evaporation of ammonia water, acceleration of the degradation reaction rate of the straws, and increase of the degradation yield of cellulose, hemicellulose and lignin.
Owner:YANGZHOU UNIV
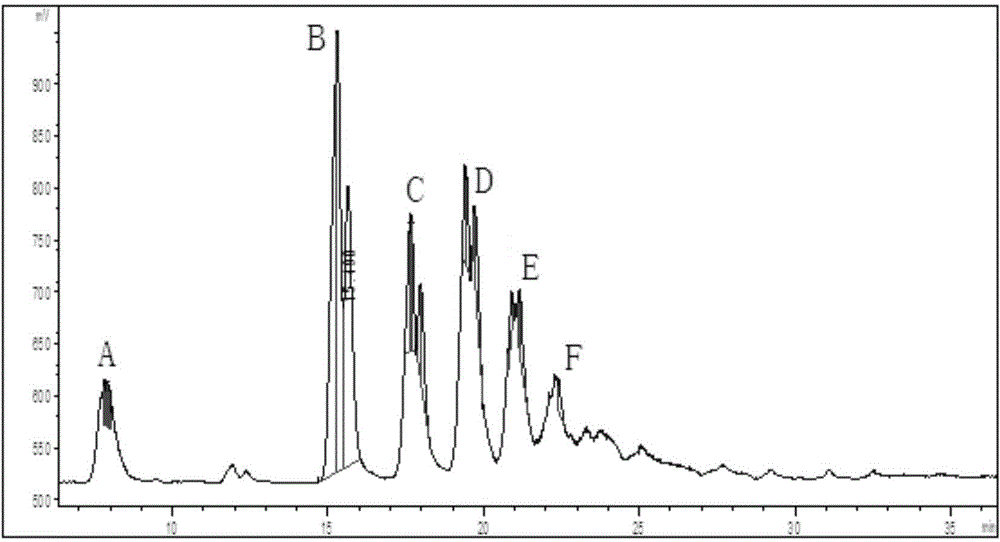
Synthetic method of azithromycin rearrangement impurity R
ActiveCN108530494AGood qualityEasy to quantitative controlSugar derivativesSugar derivatives preparationPyranoseAzithromycin
The invention belongs to the technical field of chemistry, and particularly relates to a synthetic method of an azithromycin rearrangement impurity R. The method mainly comprises the steps that (3R,4R,5S,6R,9R,10S,11S,12R,13R,15R,Z)-12-[[3,4,6-trideoxy-3-(dimethylamino)-beta-D-wood-pyranose group]oxygen]-6-ethyl-4,5-dihydroxyl-10-[(2,6-dideoxy-3-C-methyl-a-L-nuclear-pyranose group)oxygen]-3,5,9,11,13,15-hexamethyl-7,16-dioxy-2-azabicyclo[11.2.1]cetane-1-alkene-8-ketone(compound III) is subjected to acidification rearrangement, and after refining is conducted, the rearrangement impurity R withthe purity being 99.5% or above is obtained. The synthesized high-purity azithromycin rearrangement impurity R serves as an impurity standard substance of finished production detection, locating and qualification on the impurity are enhanced conveniently, and quality control over azithromycin active pharmaceutical ingredient is improved.
Owner:HEC PHARM
Combined treatment process for organophosphorus wastewater with high total phosphorus concentration
InactiveCN111875129AReduce chromaImprove absorption efficiencyWater/sewage treatment by irradiationWater treatment compoundsPtru catalystMagnesium salt
The invention relates to the field of environmental protection, in particular to a combined treatment process for organophosphorus wastewater with high total phosphorus concentration. The method comprises the steps of: pretreatment, a first-stage chemical phosphorus removal process, a flocculation phosphorus removal process, an oxidation phosphorus removal process and a second-stage chemical phosphorus removal process. Firstly, under the alkaline condition, organic phosphorus is preliminarily converted into inorganic phosphorus salt, then magnesium salt is added, a precipitation reaction is carried out to remove part of phosphorus, and then the remaining organic phosphorus pollutants can be degraded and removed through a Fenton photocatalysis combined oxidation process. By adopting the process of Fenton treatment and photocatalytic oxidation in sequence, the chromaticity of the wastewater can be reduced by utilizing Fenton treatment, the light absorption efficiency of the catalyst in the subsequent photocatalytic process can be effectively improved, and a large number of active free radicals such as superoxide anions or hydroxyl free radicals generated by residual hydrogen peroxidein the Fenton oxidation process are fully utilized; therefore, the mineralization removal rate of photocatalytic treatment of organic wastewater is further improved.
Owner:东阳市前途工业设计有限公司
Formula and production process of environment-friendly harmless air purifying agent
InactiveCN113440994APromote degradation reactionContent up to standardGas treatmentDispersed particle separationPtru catalystAir decontamination
The invention discloses a formula and a production process of an environment-friendly harmless air purifying agent. The formula consists of the following raw materials in parts by weight: 15 parts of cysteine hydrochloride, 80 parts of distilled water, 0.5 part of a food-grade surfactant T20, 4 parts of food-grade citric acid and 0.5 part of potassium sorbate. According to the invention, an edible and medicinal plant formula is adopted, so that degradation reaction with formaldehyde, benzene and sulfides can be rapidly carried out under the conditions of no illumination and no catalyst, and toxic substances such as formaldehyde and benzene are converted into safe amino acid compounds; heavy metals such as lead, cadmium, antimony and the like, arsenic, meglumine oxide, lipid peroxide, PCB (printed circuit board) and the like can be subjected to polymerization reaction to be converted into amino acid chain compounds; and the content of toxic substances such as formaldehyde and benzene can reach the standard after sufficient liquor spraying is carried out for 30 minutes, the content of heavy metal in the air reaches the standard after 24 hours, and toxic and harmful chemical components such as a heavy metal ion catalyst and a chlorine oxidizing agent which are widely used in a traditional formaldehyde removal product are not contained, so that secondary pollution is avoided.
Owner:沈阳垚采科技有限公司
Method for synthesizing azithromycin rearrangement impurity lactam
ActiveCN109575092AGood qualityEasy to quantitative controlSugar derivativesSugar derivatives preparationAzithromycinSynthesis methods
The invention belongs to the technical field of chemistry, and particularly relates to a synthesis method for azithromycin rearrangement impurity lactam. The synthesis method comprises the main step that (3R,4R,5S,6R,9R,10S,11S,12R,13R,15R,Z)-12-[[3,4,6-trideoxy-3-(dimethylamino)-beta-D-xylo-hexopyranosyl]oxy]-6-ethyl-4,5-dihydroxy-10-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl)oxy]-3,5,9,11,13,15-hexamethyl-7,16-dioxa-2-azabicyclo[11.2.1]hexadec-1-en-8-one (a compound II ) is subjected to acidification and alkalization rearrangement and then is refined so as to obtain the rearrangement impurity lactam with the purity of 99.5% or above. The synthesized high-purity azithromycin rearrangement impurity lactam serves as an impurity standard substance for finished product detection, so that positioning and nature determining on impurities is facilitated, and the quality control over azithromycin crude drugs is improved.
Owner:HEC PHARM
A kind of immobilized starter system and its preparation and application
ActiveCN109943559BThorough responseHigh speedBiofuelsMicroorganism based processesBiotechnologyEnzymatic hydrolysis
The invention relates to the technical field of biochemical industry, in particular to an immobilized fermentation system and its preparation and application; the immobilized fermentation system includes sclerotia and a composite enzyme layer coated on the surface of the sclerotium The enzyme is embedded in a porous carrier, and after immobilization, a functional environment of live bacteria and enzyme is formed, so that the substrate can complete the entire reaction process on the immobilized starter system. The preparation method is simple, and the reagent is green and environmentally friendly. It is used in The straw enzymatic hydrolysis fermentation system can significantly increase the reaction speed and speed, promote the complete reaction, and has good practicability.
Owner:SUZHOU BAIYUAN GENT CO LTD
Preparation method of inorganic biodegradable adhesive tape
PendingCN114539938AImprove antibacterial propertiesImprove structural strengthBio-packagingFilm/foil adhesivesPolymer scienceAdhesive belt
The invention discloses and relates to a preparation method of an inorganic biodegradable adhesive tape. The prepared adhesive tape is good in air permeability, has a good antibacterial effect, is degradable, is high in strength, can be used for replacing a traditional disposable plastic adhesive tape, and reduces pollution.
Owner:山联(长兴)新材料股份有限公司
A kind of synthesis method of azithromycin rearrangement impurity lactam
ActiveCN109575092BGood qualityEasy to quantitative controlSugar derivativesSugar derivatives preparationHexadecanePyranose
Owner:HEC PHARM CO LTD
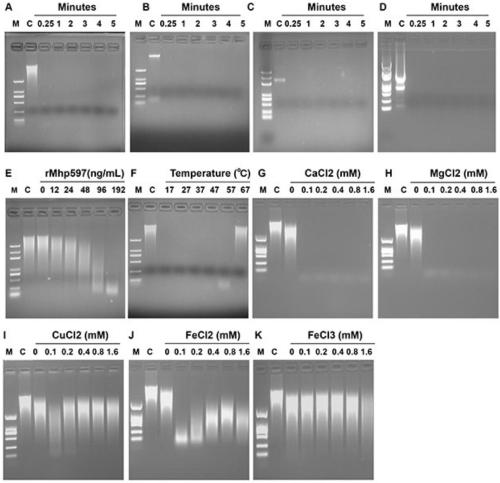
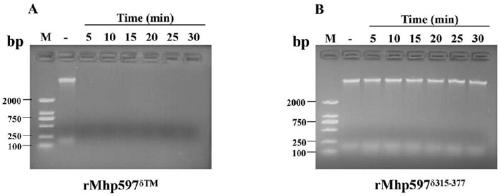
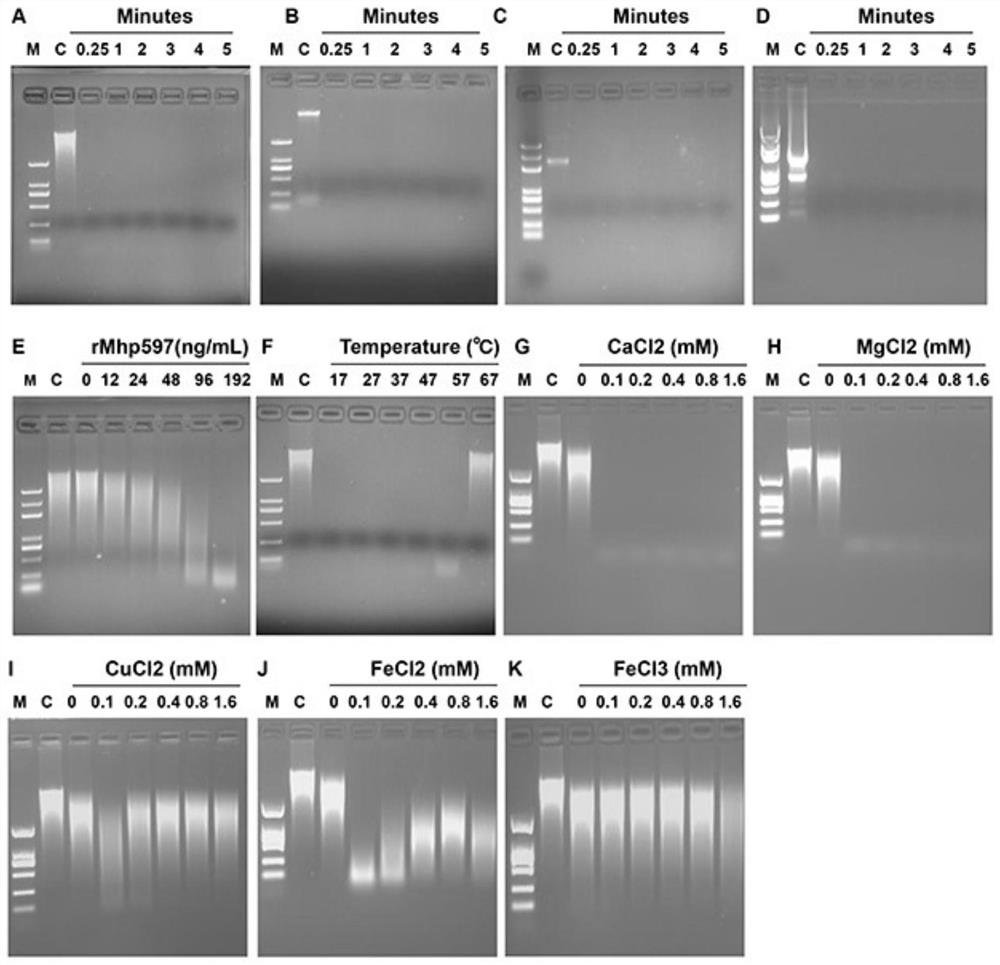
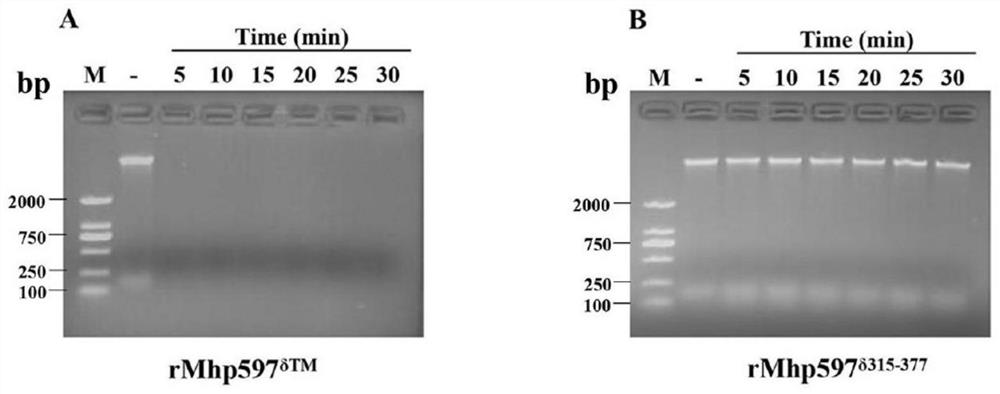
Mhp non-specific nuclease and coding gene and application thereof
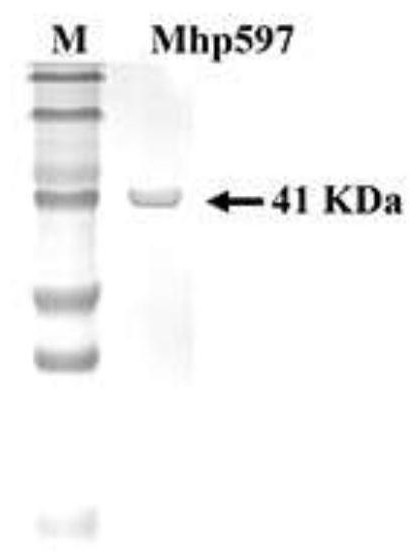
ActiveCN109234253AWide applicabilityPromote degradation reactionBacteriaHydrolasesNucleaseSingle strand dna
The present invention relates to Mhp non-specific nuclease and coding gene and application thereof. The amino acid sequence of the Mhp non-specific nuclease is shown in SEQ ID NO: 2. The Mhp non-specific nuclease provided by the invention can degrade various nucleic acid substrates, such as single-stranded DNA, double-stranded DNA, plasmid and RNA, has high enzymatic activity and high temperatureresistance, and can be widely used in the fields of biopharmaceutical, biological reagent research and development, industrialization and the like.
Owner:CHINA AGRI UNIV
Device for treating organic waste gas by applying photochemical technology
ActiveCN102861504BEfficient decompositionPromote degradation reactionDispersed particle filtrationUltraviolet lightsExhaust fumes
A device for treating organic waste gas by applying a photochemical technology comprises a high-strength security filter screen, a plasma reactor, a liquid mist humidifier, a catalytic reaction screen, an adsorption reaction screen, an ultraviolet lamp unit and an electric controller; the catalytic reaction screen and the adsorption reaction screen are subjected to the irradiation of the ultraviolet lamp unit; the organic waste gas is purified after being filtered by the high-strength filter screen, decomposed by the plasma reactor, humidified and cooled by the liquid mist humidifier, catalyzed by the catalytic reaction screen and adsorbed by the adsorption reaction screen; and the electric controller is connected to the plasma reactor and the ultraviolet lamp unit and used for controlling the starting and stopping of the plasma reactor and the ultraviolet lamp unit. The device for treating the organic waste gas by applying the photochemical technology can be used for efficiently treating industrial organic waste gas, does not produce secondary pollution residues and is lower in resource consumption and operation cost.
Owner:GUANGDONG SENYANG ENVIRONMENT PROTECTION ENG & FACILITIES
Method for preparing chitosan oligosaccharides through semi-wet method microwave treatment
A method for preparing chitosan oligosaccharides through semi-wet method microwave treatment relates to a production method of chitosan oligosaccharides. The method comprises the following steps: using chitosan gelatine powder as raw material to perform hydration and microwave treatment, dissolving in water, neutralizing, and performing membrane separation, drying and the like to prepare chitosanoligosaccharides. The method does not consume organic solvent, thus the problem of recycling solvent does not exist and the pollution is low; and as the volume of the reaction system is from 1 / 3 to 1 / 5 of that of the solution method, the production efficiency is obivously increased. The method adopts microwave treatment to promote the polysaccharide degradation reaction, thus the heating effect is greatly increased. The radiation thermal effect of microwave and the non-thermal effect of microwave are utilized to prepare the product. Meanwhile, microwave is an efficient and clean energy, thus the speed of the chemical reaction can be greatly increased. The method also adopts the membrane separation mean, thus the refining efficiency is high and the product quality is controllable.
Owner:YANGZHOU RIXING BIO TECH +1
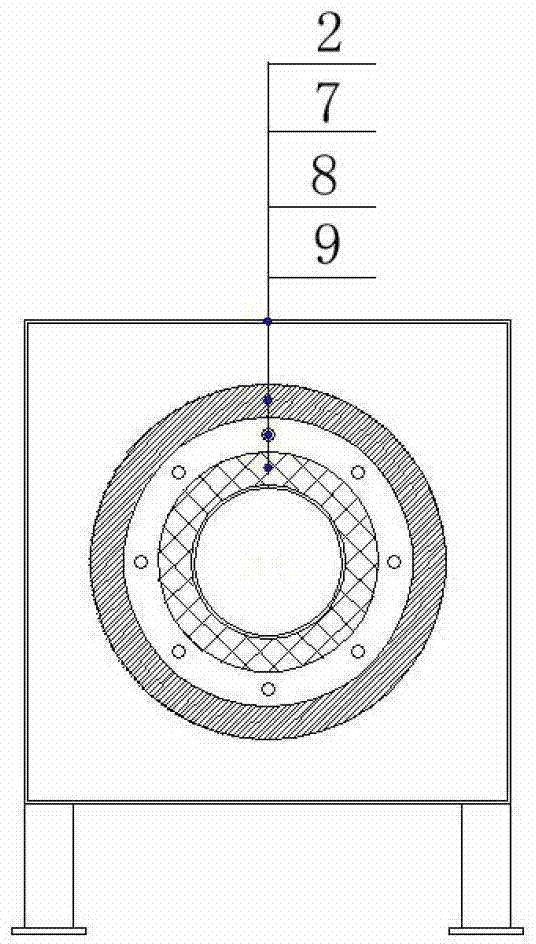
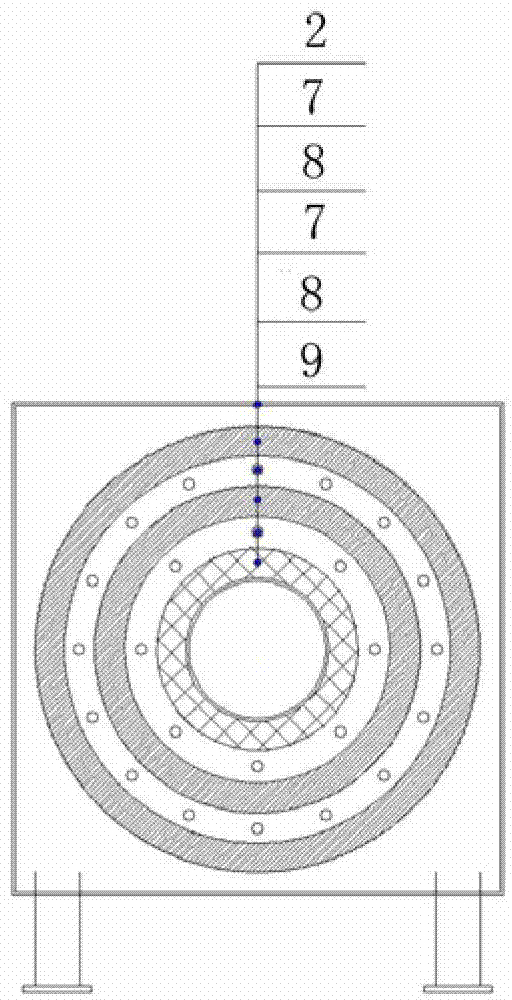
PET compound of heat aging-resistant insulating film and preparation method thereof
ActiveCN105778435BPromotes Oxygen and Water Vapor PermeationOxygen and water vapor barrierHigh heatRaw material
The invention relates to a PET compound of a heat and ageing resistant insulation film. Raw materials of the PET compound comprise, by mass, 100 parts of PET resin slices, 0.1-1.0 part of a nanoparticle nucleating agent, 10-15 parts of ultrafine nylon powder, 0.1-1.0 part of ammonium polyphosphate, 1-10 parts of pentaerythritol and 1-6 parts of triphenyl phosphite, wherein the nanoparticle nucleating agent is a silane coupling agent with the content of 4-6%, the silane coupling agent comprises a fluorine-containing silane coupling agent and an active silane coupling agent according to a mass part ratio of 0.6-1.2:1, and the ultrafine nylon powder is 600-2000 mesh nylon powder. The method has simple process flow, the dispersibility of the nanometer inorganic particle nucleating agent is easy to regulate, and the PET compound has excellent high temperature and ageing resistance.
Owner:JIANGSU YUXING FILM TECH
DMAC (dimethylacetamide) containing high-concentration organic wastewater pretreatment device
PendingCN107555683APromote degradation reactionEasy to operateWater contaminantsMultistage water/sewage treatmentAeration systemEnvironmental engineering
The invention particularly relates to a DMAC (dimethylacetamide) containing high-concentration organic wastewater pretreatment device, which comprises an acid preparation region, a degradation agent chemical adding region, a degradation reaction region, an alkaline regulating region and a discharging region, wherein the acid preparation region comprises a preparation pool and a chemical distribution pipe; the chemical distribution pipe is positioned in the preparation pool and is used for adding acid liquid for reducing the pH value of wastewater; the degradation agent chemical adding region comprises a mixing pool, a pH detecting meter and a chemical distribution pipe, the pH detecting meter is positioned in the mixing pool; the chemical distribution pipe is positioned in the mixing pooland is used for adding degradation agents; the degradation reaction region comprises a reaction pool and an aeration system which is positioned at the bottom of the inner side of the reaction pool; the alkaline regulating region comprises a middle pool and a chemical distribution pipe which is positioned in the middle pool and is used for adding alkali liquid for increasing the pH value of wastewater; the discharging region comprises a discharging pool and another pH detecting meter positioned in the discharging pool. The acid preparation region, the degradation agent chemical adding region, the degradation reaction region, the alkaline regulating region and the discharging region are in sequential arrangement. The high-concentration organic wastewater pretreatment device has the advantages that the operation is simple; the practicability is high; the treatment effect is good; the high-concentration organic wastewater pretreatment device is particularly applicable to DMAC containing high-concentration organic wastewater pretreatment.
Owner:苏州淡林环境科技有限公司
mhp non-specific nuclease and its coding gene and application
ActiveCN109234253BWide applicabilityPromote degradation reactionBacteriaHydrolasesSingle strandBiopharmaceutical
The present invention relates to Mhp non-specific nuclease and its encoding gene and application. The amino acid sequence of the Mhp non-specific nuclease is shown in SEQ ID NO:2. The Mhp non-specific nuclease provided by the invention can degrade various nucleic acid substrates, such as single-stranded DNA, double-stranded DNA, plasmid and RNA, has high enzymatic activity, and high temperature resistance, and can be widely used in biopharmaceuticals and biological reagents Research and development, industrialization and other fields.
Owner:CHINA AGRI UNIV
Method for degrading amine curing epoxy resin composite material
The invention discloses a method for degrading an amine curing epoxy resin composite material, and belongs to the technical field of solid waste recovery. The invention provides a simple and feasible degradation method aiming at the problems of high degradation temperature, long time, large catalyst dosage, material pretreatment, high cost and the like of an amine cured epoxy resin composite material in the prior art. The method comprises the following steps: by taking glacial acetic acid, water or a mixed solution of glacial acetic acid and water as a solvent, adding a nitrate catalyst to prepare a degradation solution, and adding an amine-cured epoxy resin composite material into the degradation solution for reaction; after the degradation is completed, the degradation liquid can be recycled and reused. The method has the advantages of mild reaction conditions, green and environment-friendly degradation liquid, easiness in product separation, small damage and the like.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI +1
Semi-dry microwave treating process of preparing galactomannan
ActiveCN100427496CReduce consumptionNo recycling issuesOligosaccharidesEnvironmental engineeringOligosaccharide
The present invention discloses semi-dry microwave treating process of preparing galactomanoligose. Galactomanoligose is prepared with galactomannan powder as material and through the steps of hydration, microwave treatment, water dissolving and neutralizing, membrane separating and drying. The process has short production period, high production efficiency, less pollution and low power consumption.
Owner:昆明旭日丰华农业科技有限公司
A kind of synthetic method of azithromycin rearrangement impurity r
ActiveCN108530494BGood qualityEasy to quantitative controlSugar derivativesSugar derivatives preparationPyranoseAzabicyclane
The invention belongs to the technical field of chemistry, and particularly relates to a synthetic method of an azithromycin rearrangement impurity R. The method mainly comprises the steps that (3R,4R,5S,6R,9R,10S,11S,12R,13R,15R,Z)-12-[[3,4,6-trideoxy-3-(dimethylamino)-beta-D-wood-pyranose group]oxygen]-6-ethyl-4,5-dihydroxyl-10-[(2,6-dideoxy-3-C-methyl-a-L-nuclear-pyranose group)oxygen]-3,5,9,11,13,15-hexamethyl-7,16-dioxy-2-azabicyclo[11.2.1]cetane-1-alkene-8-ketone(compound III) is subjected to acidification rearrangement, and after refining is conducted, the rearrangement impurity R withthe purity being 99.5% or above is obtained. The synthesized high-purity azithromycin rearrangement impurity R serves as an impurity standard substance of finished production detection, locating and qualification on the impurity are enhanced conveniently, and quality control over azithromycin active pharmaceutical ingredient is improved.
Owner:HEC PHARM CO LTD
An amphiphilic nanotio 2 Powder catalyst and its preparation method and use method
ActiveCN105363495BAvoid reunion exacerbationInhibit sheddingWater/sewage treatment by irradiationOrganic-compounds/hydrides/coordination-complexes catalystsHigh concentrationWastewater
Belonging to the technical field of catalyst preparation and application, the invention discloses an amphiphilic nano TiO2 powder catalyst, a preparation method and a use method thereof. According to the invention, liquid phase impregnation is firstly adopted for modification of TiO2, two surface modification substances are introduced to make the modified TiO2 have a contact angle of 75-85 degrees and an amphiphilic surface; then the amphiphilic catalyst powder is subjected to ultrasonic dispersion in high concentration organic wastewater so as to be more conducive to formation of a stable Pickering emulsion, and photocatalytic degradation of high concentration organic wastewater is carried out under simulated solar illumination to achieve the purpose of wastewater treatment. The amphiphilic nano TiO2 powder catalyst provided by the invention is practical and is convenient to operate, can reach efficient degradation of wastewater based on low treatment cost, and can reach a degradation rate up to 99%.
Owner:ZHONGBEI UNIV
Targeted fusion protein against HBV replication and construction method thereof
ActiveCN107056947BStrong inhibitory effect on replicationPromote degradation reactionPolypeptide with localisation/targeting motifPeptide/protein ingredientsHepatitis B immunizationHbv replication
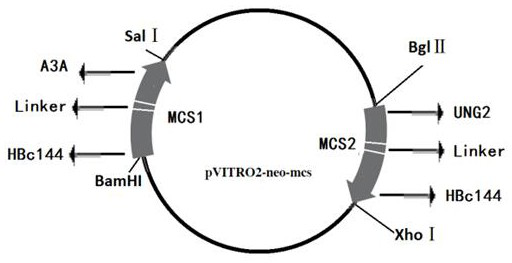
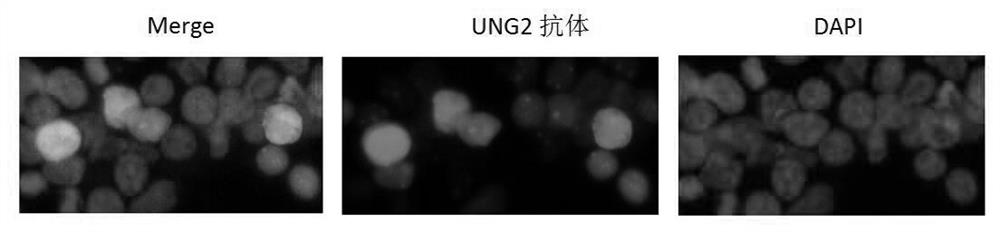
The invention relates to an anti-HBV replication targeting fusion protein and a construction method thereof. Utilizing the characteristic that the C-terminal region (CTD) of the hepatitis B virus core protein HBc can specifically bind to HBV cccDNA, this peptide is used as a guide molecule targeting cccDNA, which is denoted as HBc144; A3A‑HBc144 coding gene sequence and HBc144 were synthesized ‑UNG2 coding gene sequence, and clone these two segments into the multiple cloning sites MCS1 and MCS2 of the eukaryotic expression vector pVITRO2‑neo‑mcs to construct a recombinant double expression vector Pvitro2‑A3A‑HBc / HBc‑UNG2, The recombinant vector can simultaneously express two fusion proteins in hepatocytes, that is, A3A-HBc144 and HBc144-UNG2. The present invention realizes the targeting of A3A and UNG2 to HBV cccDNA, which is more effective than the vector expressing A3A or A3A-HBc alone. Strong inhibitory effect on virus replication.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com