Controllable preparation method of non-noble metal monatomic catalyst
A non-precious metal and catalyst technology, applied in the field of controllable preparation of non-precious metal single-atom catalysts, can solve problems such as lack of solutions, and achieve the effects of improving atom utilization, high catalytic activity, and strong stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
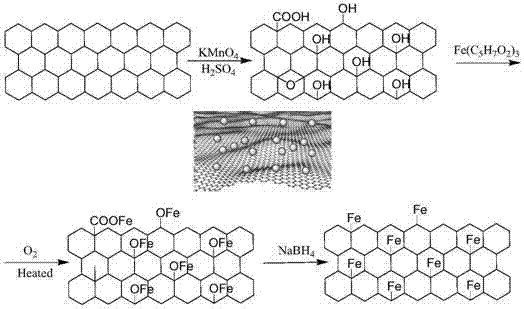
[0035] Such as figure 1 As shown, potassium permanganate is used to oxidize graphene to prepare graphene oxide: commercial graphite powder is treated in a mixture of sulfuric acid and potassium permanganate by Hummers method to obtain graphene oxide. The obtained brown product is a graphite flake with derivatized carboxylic acid groups on the edge and mainly phenolic hydroxyl groups and epoxy groups on the plane, which is exfoliated into graphene oxide by ultrasonic or high-shear vigorous stirring. Oxygen-containing functional groups such as hydroxyl, carboxyl and epoxy groups were prepared on graphene.
[0036] Weigh 0.0255g of graphene oxide and pour it into a beaker, add 10ml of ethanol, and sonicate for 2 hours. Weigh 0.0257g of graphene oxide and pour it into a beaker, add 10ml of methanol, and sonicate for 2 hours. Weigh 2.7304g Fe(C 5 h 7 o 2 ) 3 ·7H 2 O was put into a round-bottomed flask, and 25ml of deionized water was added to adjust the pH to 6.0. Start the ...
Embodiment 2
[0038] Weigh 0.8772g CuSO 4 ·5H 2O and 1.8599 g FeCl 3 ·7H 2 O was put into a round-bottomed flask, added 25ml of deionized water, adjusted to pH 4.0, started the electric mixer, and stirred at 20°C for 30 minutes. Then the graphene oxide dispersion was mixed with the metal salt solution and sonicated at 60 °C for 2 h. Weigh 0.2099gNaBH 4 Dissolve in 10ml deionized water, NaBH 4 The solution was dropped dropwise into the mixed solution of the graphene oxide dispersion and the metal salt, reacted for 0.5 hour, and the obtained product was centrifuged and washed three times with deionized water. Finally put it into a freeze dryer to freeze dry. The metal atom loading capacity of the obtained single-atom catalyst is 1%, and the utilization efficiency of copper and iron metal atoms can reach 99.5%.
Embodiment 3
[0040] Weigh 0.0257g of graphene oxide and pour it into a beaker, add 10ml of methanol, and sonicate for 2 hours. Weigh 2.5304gFeCl 3 ·7H 2 O was put into a round-bottomed flask, and 25ml of deionized water was added to adjust the pH to 6.0. Start the electric mixer and stir for 30 minutes at 20°C. Then the graphene oxide dispersion was mixed with the metal salt solution and sonicated at 60 °C for 2 h. Weigh 0.2099g KBH 4 Dissolve KBH in 10ml deionized water 4 The solution was dropped dropwise into the mixed solution of the graphene oxide dispersion and the metal salt, reacted for 0.5 hour, and the obtained product was centrifuged and washed three times with deionized water. Finally put it into a freeze dryer to freeze dry. The metal atom loading capacity of the obtained single-atom catalyst is 5%, and the iron metal atom utilization efficiency can reach 100%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com