Regulation and control method of expression in chimeric antigen receptor
A chimeric antigen receptor and expression system technology, applied in the field of biomedical transformation, can solve problems such as patient death and cytokine storm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1. Design of Expression Vectors for Unnatural Amino Acid-Dependent Recombinant Chimeric Antigen Receptors
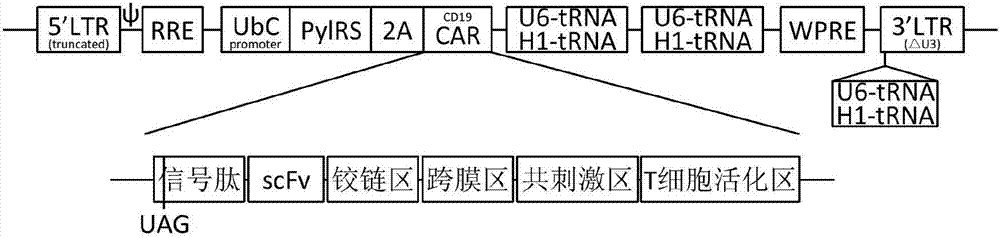
[0052] The present invention selects the currently researched and most widely used CD19-CAR molecule as an example, uses the plasmid pUltra as the backbone, and constructs the lentiviral transfection plasmid of the non-natural amino acid-dependent recombinant chimeric antigen receptor as follows ( figure 1 ):
[0053] Lentiviral Transfer Plasmids: Lenti-RS / tRNA / CAR
[0054] 5'LTR(truncated)-ψ-RRE-UbC promoter-PylRS-2A-CD19CAR-(U6-tRNA-H1-tRNA)*2-3'LTR [△U3 (U6-tRNA-H1-tRNA)-R- U5]
[0055] Among them, the CAR molecule UAG—Signal peptide-scFv-hinge-transmembrane domain-4-1BB-CD3 zeta.
[0056] The plasmid construction was synthesized by Beijing Aoke Biotechnology Co., Ltd. and sequenced to confirm the correct sequence.
Embodiment 2
[0057] Embodiment 2, the packaging of lentiviral vector
[0058] Inoculate 5 × 10 in gelatin-precoated 15-cm dishes 6 293T cells at 37 °C, CO 2 Incubate overnight in an incubator. When the confluency reaches 70%, prepare the plasmid for transfection. Two hours before transfection, replace with fresh serum-free medium for transfection.
[0059] Take out the transfection reagent 100 μM PEI (Polyethyleneimine) from the refrigerator, and heat it in a water bath at 60°C for 15 minutes until it is completely dissolved. Take out the plasmids Lenti-RS / tRNA / CAR, pMDL g / pRRE, pRSV-Rev, pMD2.G from the refrigerator and dissolve at room temperature.
[0060] Preparation of PEI / DNA complex: take 2ml PBS, add 10μg Lenti-RS / tRNA / CAR, 5μg pMDL g / pRRE, 2.5μg pRSV-Rev, 2.5μg pMD2.G, after blowing and mixing well, add 18μl 100μM PEI, Immediately inhale and mix well, and let stand at room temperature for 10 minutes. Add the PEI / DNA complex obtained after standing still to the cell culture ...
Embodiment 3
[0062] Example 3, lentivirus-mediated cell transfection
[0063] Add Polybrene to an appropriate volume of serum-free RPMI 1640 to a final concentration of 8 μg / ml, use the culture medium to resuspend the Jurkat cells collected by centrifugation, and dilute 5×10 5 The density per well was seeded in a 6-well plate. According to the virus titer and cell number, add the lentivirus concentrate with MOI=10, shake the culture plate gently to mix. Placed at 37°C, 5% CO 2 Culture in an incubator, and change to RPMI 1640 medium containing 10% FBS after 16-24 hours to continue the culture.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com