A Spherical Nano-zno/zncr with Efficient Hydrogen Production 2 o 4 Preparation method of composite photocatalyst
A composite photocatalysis and photocatalyst technology, which is applied in the direction of physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the problem of the small number of heterogeneous nodes and the agglomeration of photocatalysts , Photocatalytic activity is limited to increase and other issues, to achieve the effect of increasing heterojunction, increasing specific surface area, controlling grain growth and uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
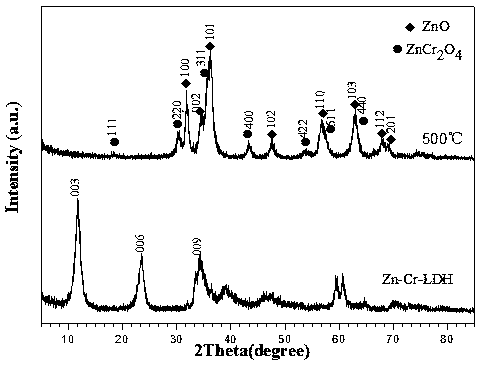
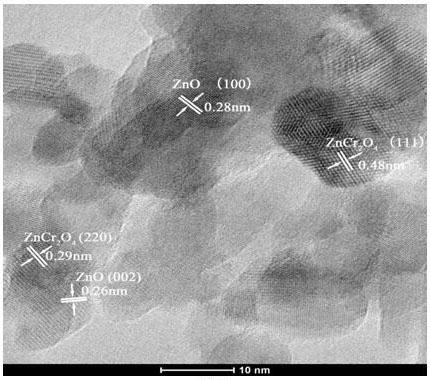
Image
Examples
Embodiment 1
[0028] (1) Weigh 0.04mol zinc nitrate and 0.02mol chromium nitrate and add them to deionized water, stir magnetically until completely dissolved to form a mixed nitrate solution, and the total concentration of metal ions is controlled within 0.1mol / L;
[0029] (2) Take by weighing 0.22g small molecule waxy substance and add in the mixed nitrate solution that step (1) makes as dispersant, add 0.56 g urea to dissolve completely under magnetic stirring;
[0030] (3) Transfer the reaction mixture obtained in (2) into a round bottom flask, reflux at 120 °C for 4 h under magnetic stirring, and then stand at the same temperature for 4 h to obtain the microcrystalline precursor;
[0031] (4) Transfer the precursor obtained in (3) to a hydrothermal reactor for hydrothermal aging reaction. The hydrothermal aging temperature is 120 °C, the reaction time is 8 h, and the reactants are naturally cooled to room temperature;
[0032] (5) The reaction product obtained in step (4) was subjected...
Embodiment 2
[0035] (1) Weigh 0.06mol of zinc nitrate and 0.02mol of chromium nitrate and add them into ionized water, stir magnetically until completely dissolved to form a mixed nitrate solution, and the total concentration of metal ions is controlled within 0.1mol / L;
[0036] (2) Take by weighing 0.44g small molecule waxy substance and add in the mixed nitrate solution that step (1) makes as dispersant, add 0.79 g urea to dissolve completely under magnetic stirring;
[0037] (3) Transfer the reaction mixture obtained in (2) into a round bottom flask, reflux at 110 °C for 6 h under magnetic stirring, and then stand at the same temperature for 6 h to obtain a microcrystalline precursor;
[0038] (4) Transfer the precursor obtained in (3) to a hydrothermal reaction kettle for hydrothermal aging reaction. The hydrothermal aging temperature is 110°C, the reaction time is 12h, and the reactants are naturally cooled to room temperature;
[0039] (5) Vacuum filter the reaction product obtained ...
Embodiment 3
[0042] (1) Weigh 0.08mol of zinc nitrate and 0.03mol of chromium nitrate into deionized water, stir magnetically until completely dissolved to form a mixed nitrate solution, and the total concentration of metal ions is controlled within 0.1mol / L;
[0043] (2) Take by weighing 0.66g small molecule waxy substance and add in the mixed nitrate solution that step (1) makes as dispersant, add 1.12 g urea to dissolve completely under magnetic stirring;
[0044] (3) Transfer the reaction mixture obtained in (2) into a round-bottomed flask, reflux at 100 °C for 8 h under magnetic stirring, and then stand at the same temperature for 10 h to obtain a microcrystalline precursor;
[0045] (4) Transfer the precursor obtained in (3) to a hydrothermal reactor for hydrothermal aging reaction. The hydrothermal aging temperature is 120 °C, the reaction time is 8 h, and the reactants are naturally cooled to room temperature;
[0046] (5) Vacuum filter the reaction product obtained in step (4), wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com