American cockroach peptide B with anti-hepatoma activity and application of American cockroach peptide B
An anti-liver cancer, cockroach technology, applied in the field of medicine, can solve the problems of hypersensitivity, reduce the efficacy of drugs, can not meet the medical market, etc., and achieve the effect of small dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] American cockroach peptide B exists in the medicinal materials of Periplaneta americana. The extraction method of the American cockroach peptide B comprises the following steps: after the Periplaneta americana medicinal material is extracted with a solvent, then repeatedly extracted and separated by macroporous resin adsorption, dextran gel column chromatography, high performance liquid chromatography, etc., A sample containing cockroach peptide B was obtained.
[0035] Sangon Biology (Shanghai) Co., Ltd. was entrusted to prepare meicockatipe peptide B for pharmacodynamic evaluation test. The physicochemical properties of described melilay peptide B are as follows:
[0036] Number of amino acid residues: 8 1 character: DDLRGDND 3 characters: Asp-Asp-Leu-Arg-Gly-Asp-Asn-Asp Molecular weight: 918.87g / mol Isoelectric point: 3.4 Net charge at pH=7: -3.0 Average Hydrophilicity: 1.7 Hydrophilic residue ratio: 75%
...
Embodiment 2
[0040] 1. Cell culture of human liver cancer HepG2 cell line
[0041] Human liver cancer HepG2 cell line adopts high-glucose DMEM complete medium containing 10% fetal bovine serum, placed in 5% CO 2 , Cultured in a 37°C incubator, and the cells in the logarithmic growth phase were taken for experiments.
[0042] 2. MTT method to detect the survival rate of human liver cancer cell HepG2 cells after treatment
[0043] HepG2 cell seed plate (1×10 5 mL -1, 100 μL) were inoculated in a 96-well plate and cultured for 24 h. Concentrations were configured into cockroach peptide B solutions with concentrations of 6.25, 12.5, 25, 50, 100, and 150 μg / mL, and the above-mentioned 6 concentrations of cockroach peptide B solutions were used for intervention, and 6 replicate wells were set for each concentration. 200 μL, set thalidomide (concentration: 100 μg / mL, 200 μg / mL) positive control group and blank control group at the same time, continue to culture for 24 hours. The absorbance v...
Embodiment 3
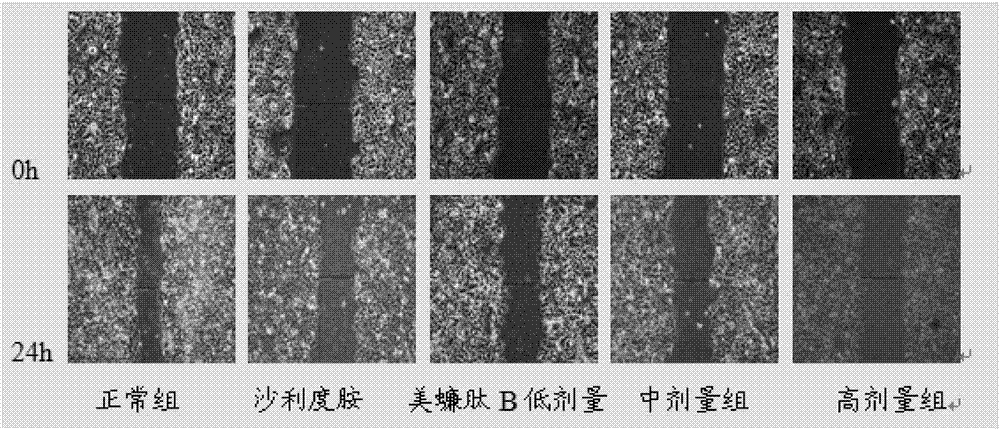
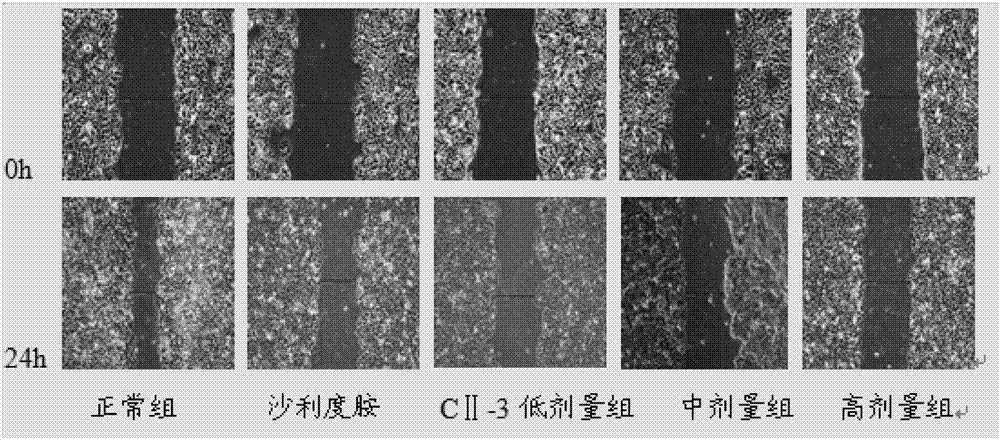
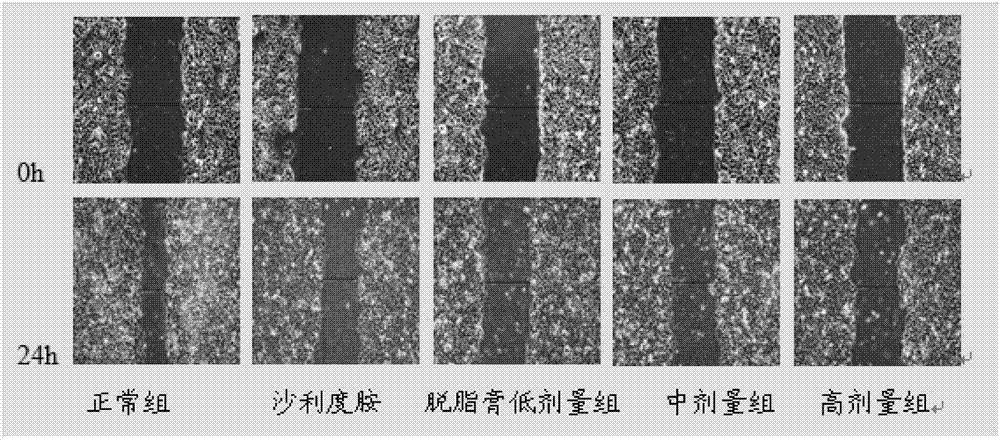
[0056] Inhibition of Migration of HepG2 Cells by Peptide B in Cell Scratch Test
[0057] Experimental grouping: blank control group, thalidomide control group (concentration of 100 μg / mL, 200 μg / mL), meicockatin B concentration set to low, medium and high dose groups (25 μg / mL, 50 μg / mL, 100 μg / mL mL).
[0058] HepG2 cells were routinely digested at a density of 2×10 5 mL -1 Inoculate in a 6-well plate, 2 mL per well. After culturing for 24 hours, use a 200 μL pipette tip to draw a straight line at the bottom of the 6-well plate, wash with PBS 2 to 3 times, and add drugs according to the experimental groups. Take pictures of the scratches at 0h and 24h respectively, and measure the width of the scratches. The above experiment was repeated three times, and the average value was taken to calculate the scratch healing rate. Scratch healing rate=(0h scratch width-24h scratch width) / 0h scratch width×100%.
[0059] Data processing adopts SPSS17.0 statistical software and Origi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com