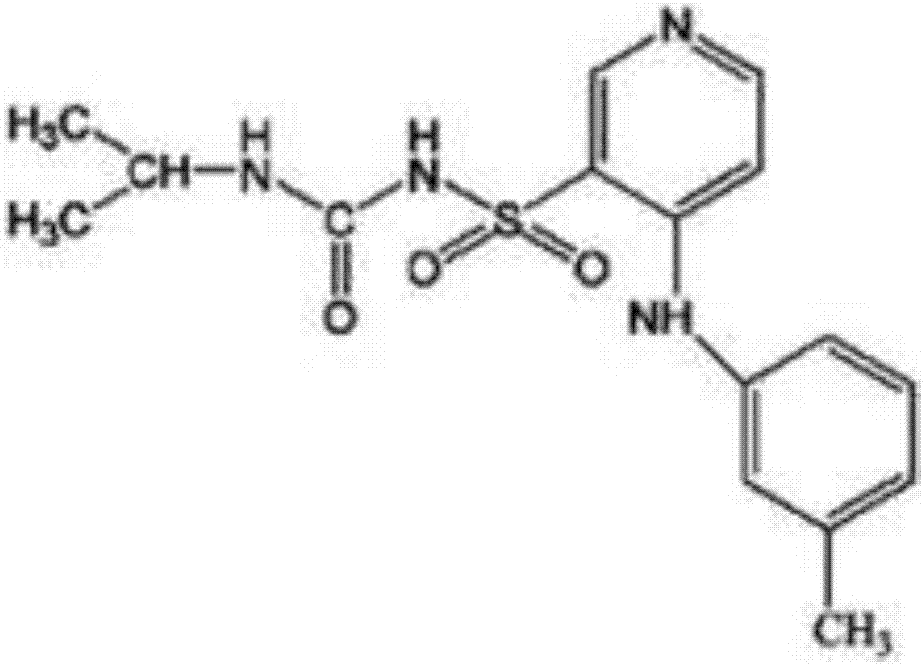
Sublingual oral preparation of torasemide
An oral preparation, torasemide technology, applied in the medical field, can solve the problems of gastrointestinal irritation, obvious first-pass effect, narrow adaptability, etc., to speed up the onset time, expand the contact area, and reduce the amount of use. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
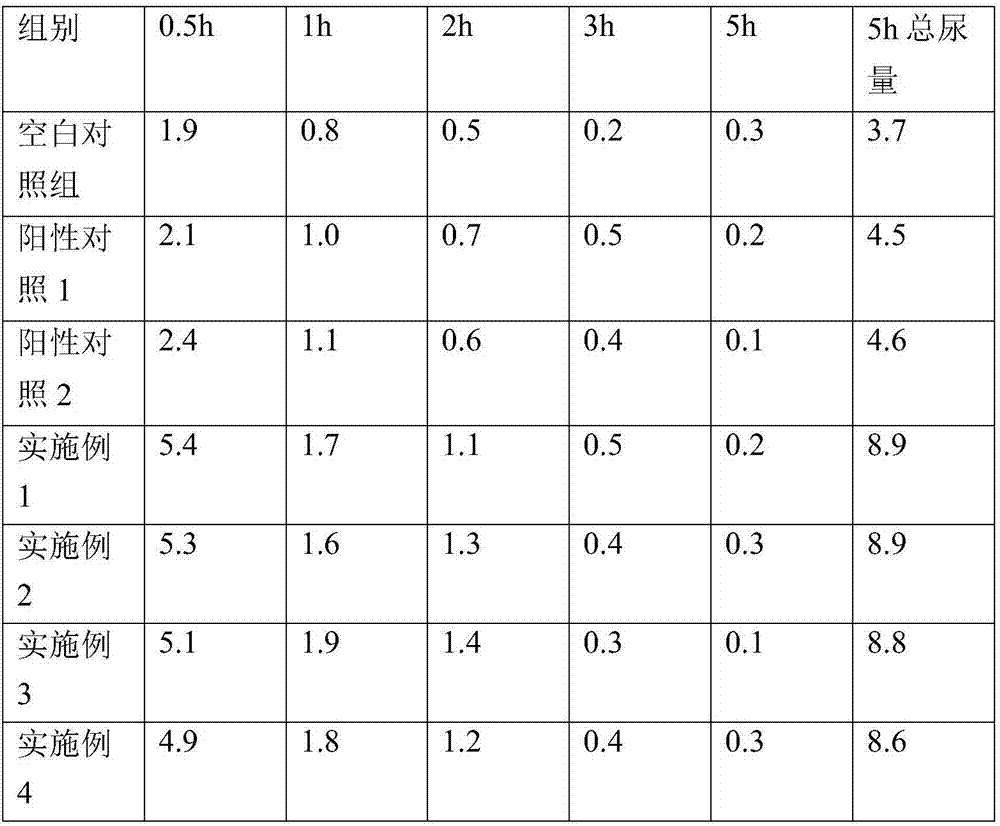
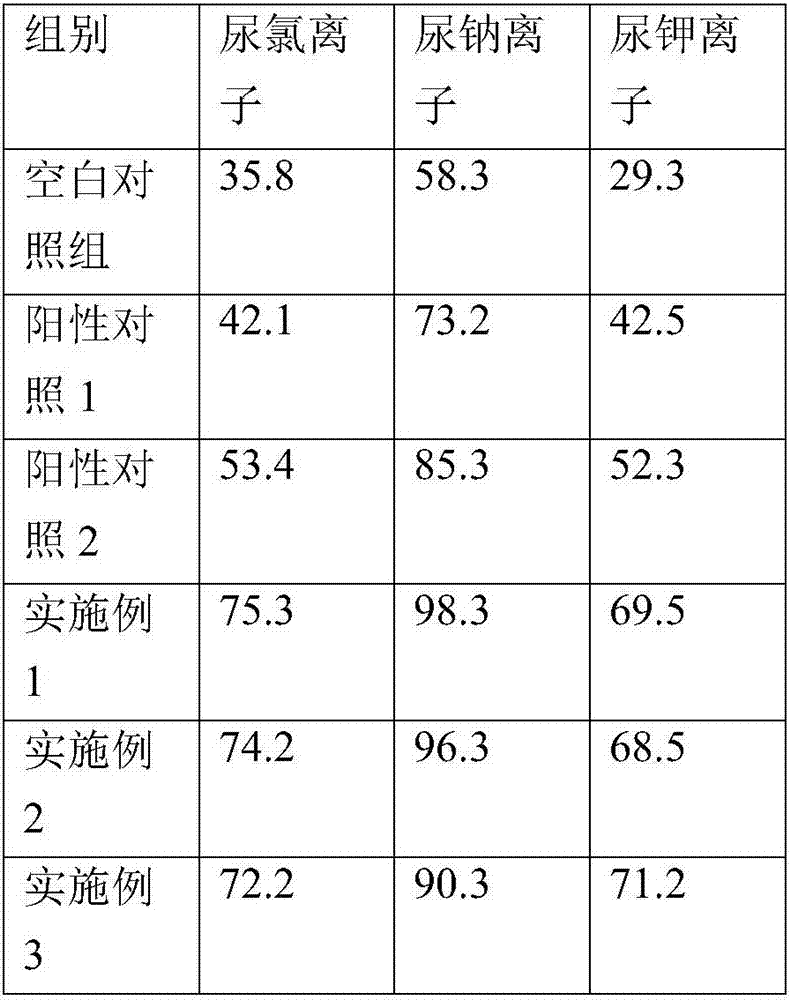
[0023] The sublingual oral preparation comprises the following raw materials in parts by weight: 25 parts by weight of torasemide, 60 parts by weight of chitosan, 45 parts by weight of crospovidone, and 170 parts by weight of mannitol, which are prepared by mixing and pressing into tablets.
Embodiment 2
[0025] The sublingual oral preparation comprises the following raw materials in parts by weight: 25 parts by weight of torasemide, 50 parts by weight of cyclodextrin, 35 parts by weight of croscarmellose sodium, and 190 parts by weight of mannitol, which are mixed and compressed into tablets.
Embodiment 3
[0027] Sublingual oral preparations, comprising the following raw materials in parts by weight: 25 parts by weight of torasemide, 80 parts by weight of oleic acid, 25 parts by weight of crospovidone, 20 parts by weight of croscarmellose sodium, and 150 parts by weight of sucrose , prepared by mixing tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com