Pharmaceutical composition for treating chronic nephritis
A technology of chronic nephritis and composition, applied in the field of medicine, can solve problems such as poor prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0022] The characterization of experimental example 1 medicine of the present invention
[0023]
[0024] 1 H NMR (400MHz, CDCl 3 )δ8.14(d, J=2.4Hz, 1H), 7.7(dd, J=8.4, 2.4Hz, 1H), 7.70(d, J=8.4Hz, 1H), 7.64(d, J=2.4Hz, 1H), 7.23-7.16(m, 2H), 5.17(s, 2H), 2.92(t, J=6.4Hz, 2H), 2.65(dd, J=13.2, 6.0Hz, 2H), 2.60(s, 3H ),2.16-2.10(m,2H).
experiment example 2
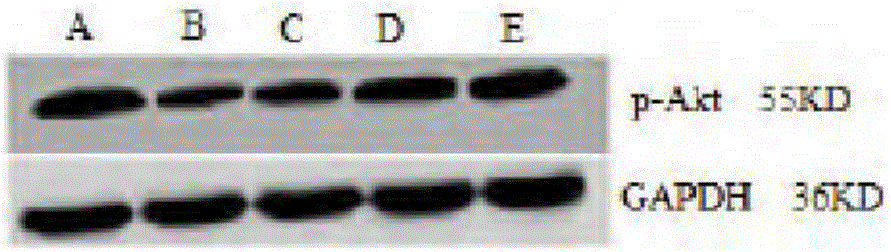
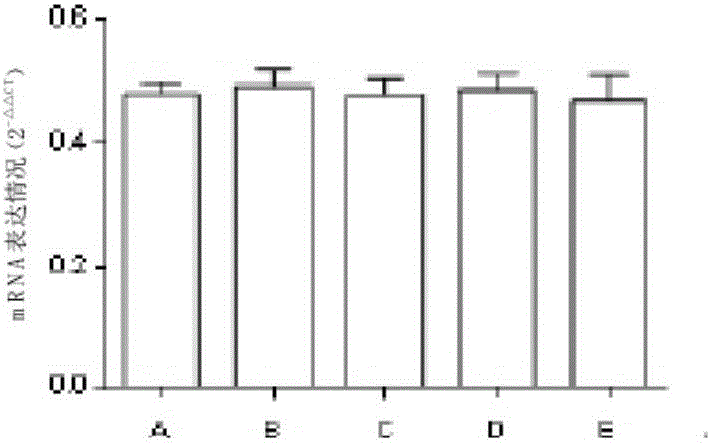
[0025] Experimental example 2 the effect of medicine treatment chronic nephritis of the present invention
[0026] Animals and Groups
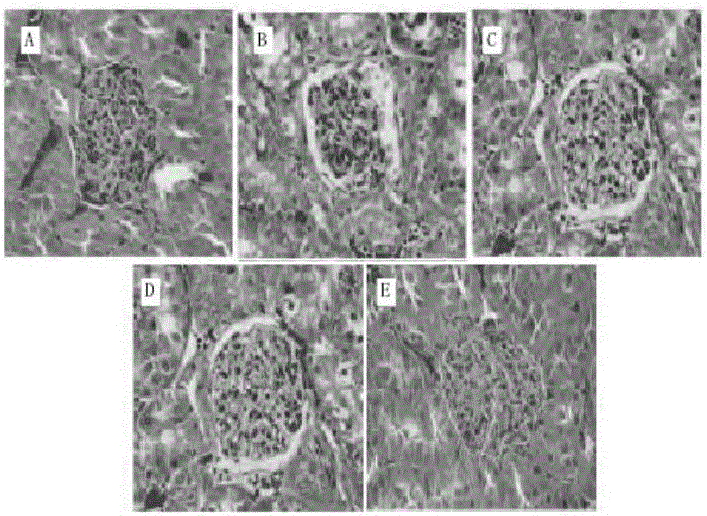
[0027] Select healthy and clean male SD rats with a body weight of 120-150 g, and feed them with an adaptive common diet at room temperature for 1 week. The rats are divided into blank group, model group, and high, medium and low dose groups of the drug of the present invention by random number table method , 10 each. The urine protein was qualitatively negative for 2 times (test strip method) and then the model was established. Rats were sacrificed at the end of the 16th week.
[0028] modeling
[0029] Reference literature (Zhang Xiaoqiang, Liu Pinli, Li Mengfang, et al. Development of animal models of allergic purpura epilepsy. Chinese Journal of Traditional Chinese Medicine, 2011, 26(10): 2319-2324.): Model group and the drug of the present invention have high, medium and low The dose group used the BSA+SFB method to create the IgA nep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com