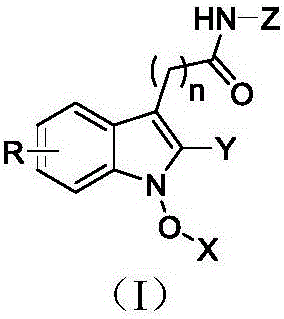
Indole compound with antivirus activity in radix isatidis and derivative of indole compound
A compound and selected technology, applied in the field of medicine, can solve the problems of undiscovered anti-HIV and anti-influenza virus activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
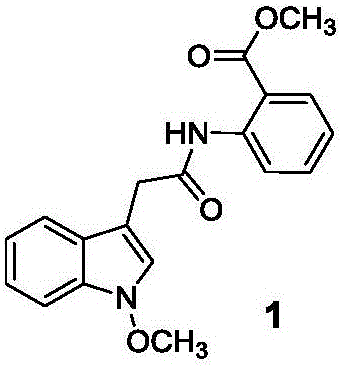
[0228] Embodiment 1: Preparation of 2-(1-hydroxyl-1H-indol-3-yl)-N-phenylacetamide
[0229]
[0230] In the first step, weigh indole acetic acid (1.05g), add dichloromethane (20mL) to suspend and stir, add EDCI (1.27g) at room temperature, stir and dissolve; add aniline (0.62g), DMAP (0.15g), Stir the reaction at room temperature for 3 h; add 2N hydrochloric acid and stir rapidly for 10 min, and separate the phases; add saturated brine (10 mL) to the organic phase and stir for 10 min, and separate the phases; -indol-3-yl)-N-phenylacetamide.
[0231] In the second step, 2-(1H-indol-3-yl)-N-phenylacetamide was dissolved in trifluoroacetic acid (10mL), triethylsilane (1.74g) was added, and the reaction was refluxed at 60°C for 3h; the reaction solution Recover the solvent, add ethyl acetate (30mL), saturated aqueous sodium bicarbonate (30mL), stir rapidly for 15min, and separate the phases; extract the aqueous phase with ethyl acetate (30mL), and separate the phases; combine ...
Embodiment 2
[0233] Embodiment 2: Preparation of ethyl-2-[2-(1-hydroxyl-1H-indol-3-yl)acetamide]benzoate
[0234]
[0235] The first step, weigh indole acetic acid (1.05g), add dichloromethane (20mL) to suspend and stir, add EDCI (1.27g) at room temperature, stir to dissolve; add ethyl anthranilate (1.09g), DMAP (0.15g), stirred at room temperature for 3h; added 2N hydrochloric acid and stirred quickly for 10min, and separated the phases; added saturated brine (10mL) to the organic phase and stirred for 10min, and separated the phases; the organic phase was removed under reduced pressure at 40°C to obtain light reddish-brown oil Crude ethyl-2-[2-(1H-indol-3-yl)acetamide]benzoate.
[0236]In the second step, ethyl-2-[2-(1H-indol-3-yl)acetamide]benzoate was dissolved in trifluoroacetic acid (10mL), triethylsilane (1.74g) was added, 60 Reflux reaction at ℃ for 3h; the solvent was recovered from the reaction solution, ethyl acetate (30mL) and saturated aqueous sodium bicarbonate (30mL) wer...
Embodiment 3
[0238] Embodiment 3: the preparation of methyl-3-[2-(1-hydroxyl-1H-indol-3-yl)acetamide]-4-methylbenzoate
[0239]
[0240] The first step, weigh indole acetic acid (1.05g), add dichloromethane (20mL) to suspend and stir, add EDCI (1.27g) at room temperature, stir to dissolve; add 4-methyl-3-amino-benzoic acid methyl Ester (1.09g), DMAP (0.15g), stirred at room temperature for 3h; added 2N hydrochloric acid and stirred rapidly for 10min, and separated the phases; added saturated brine (10mL) to the organic phase and stirred for 10min, and separated the phases; the organic phase was removed under reduced pressure at 40°C , to obtain crude methyl-3-[2-(1H-indol-3-yl)acetamide]-4-methylbenzoate as light reddish brown oil.
[0241] In the second step, methyl-3-[2-(1H-indol-3-yl)acetamide]-4-methylbenzoate was dissolved in trifluoroacetic acid (10mL), and triethylsilane ( 1.74 g), reflux at 60°C for 3 h; the solvent was recovered from the reaction solution, ethyl acetate (30 mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com