Resource utilization method of 2,4-diaminobenzenesulfonic acid and waste water produced by 2,4-diaminobenzenesulfonic acid salt
A technology for the production of diaminobenzenesulfonic acid and waste water, which is applied in chemical instruments and methods, organic chemistry, and the preparation of organic compounds. It can solve the problems of unreasonable utilization and waste of resources, and achieve the effect of improving comprehensive utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
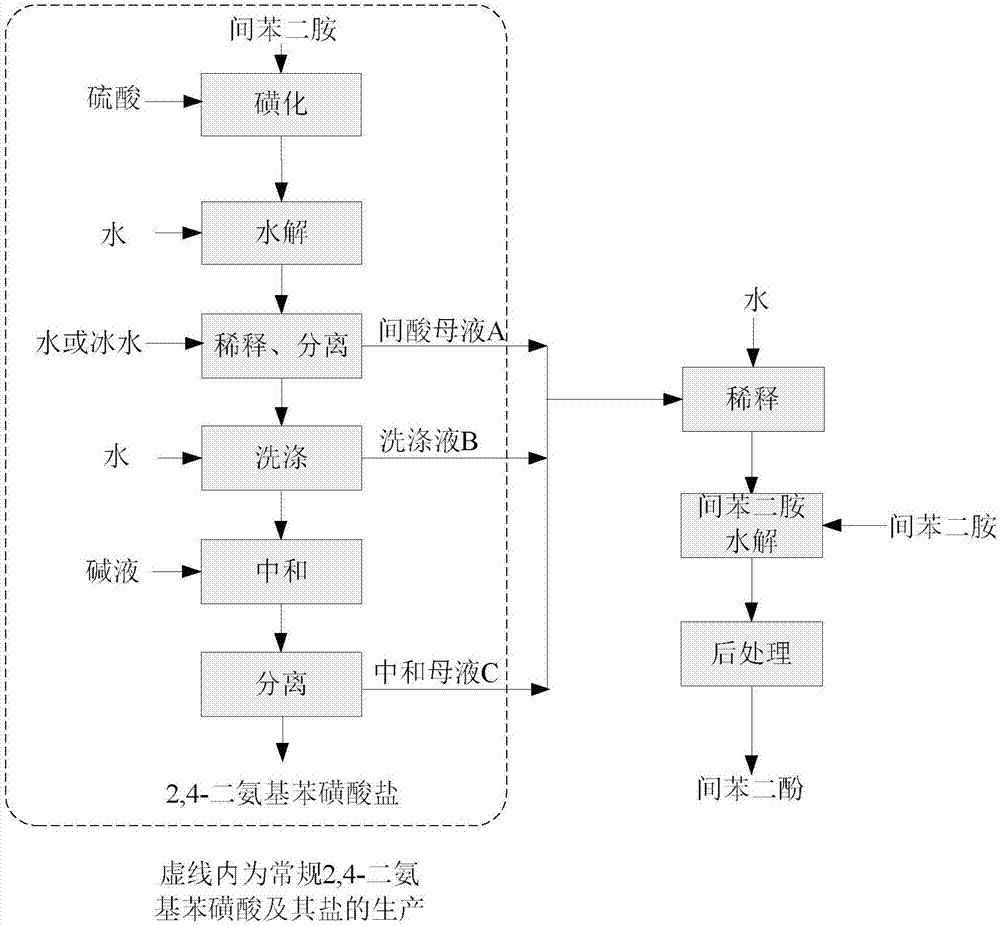
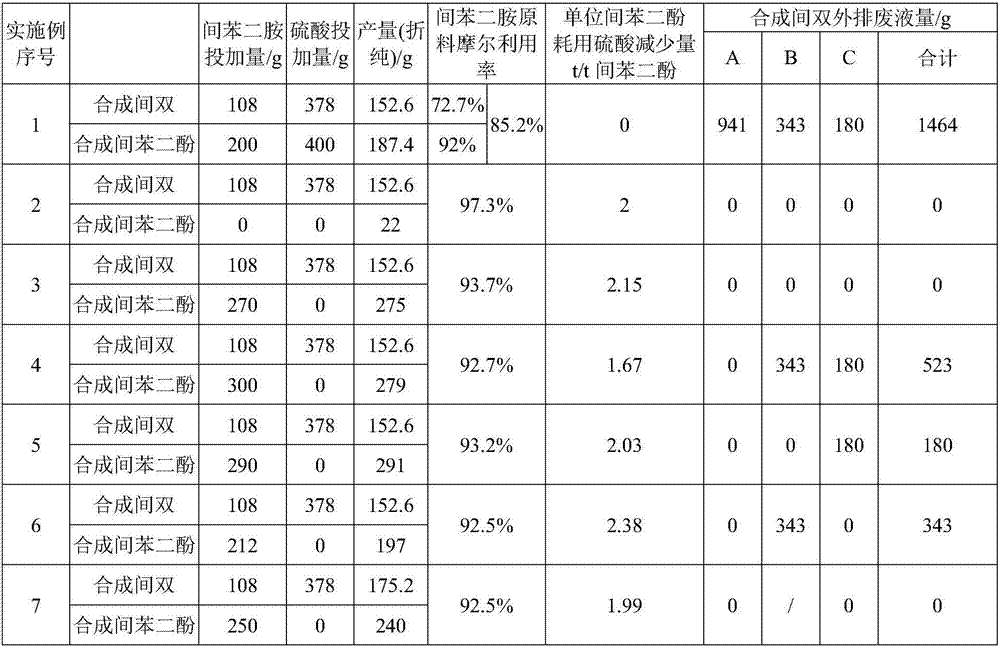
[0022] (1) The synthesis of 2,4-diaminobenzenesulfonic acid and its salts adopts a conventional process: add 108g of m-phenylenediamine to 378g of 98% sulfuric acid, control the temperature of the addition process within 120°C, and form a uniform salt solution after the addition, and raise the temperature To 150°C, slowly add 271.3g of 50% fuming sulfuric acid for sulfonation, and keep warm for 4h after the addition. After cooling the sulfonated solution to 130°C, slowly add 32.4g of water, keep it warm for 20min, then complete the hydrolysis and cool down to room temperature. Slowly add the cooled hydrolyzate into 550g of ice water, filter at about 15°C, and separate the filter cake and 941g of inter-acid mother liquor A. The filter cake was washed with 200 g of water, and after washing, the meta-acid solid and 343 g of washing liquid B were separated, the meta-acid yield was 74.6%, and the meta-acid purity (HPLC) was 99.5%. Add 32% sodium hydroxide aqueous solution to the m...
Embodiment 2
[0025] (1) according to the method for step (1) described in embodiment 1, synthesizing meta-bis, obtain 941g meta-acid mother liquor A, 343g washing liquid B, 180g neutralize mother liquor C three strands of waste water;
[0026] (2) it is the dilute acid solution that the acidity is 11% that step (1) is mixed with 3900g water preparation acidity mother liquor A, washing solution B, neutralization mother liquor C three waste waters that step (1) obtains, do not add m-phenylenediamine, directly dilute The acid solution was preheated to 240°C and added to the reactor for hydrolysis for 240 minutes; after the reaction, 26g of resorcinol (reduced pure) was obtained by extraction and rectification, the product purity of resorcinol (HPLC) was 99.2%, and the mole of m-phenylenediamine raw material The utilization rate is 97.3%.
Embodiment 3
[0028] (1) according to the method for step (1) described in embodiment 1, synthesizing meta-bis, obtain 941g meta-acid mother liquor A, 343g washing liquid B, 180g neutralize mother liquor C three strands of waste water;
[0029] (2) mixing the meta-acid mother liquor A obtained in step (1) with 3900g water to prepare acidity is a dilute acid solution of 11%, adding 270g of molten m-phenylenediamine to prepare a hydrolysis raw material solution; preheating the prepared hydrolysis raw material solution After reaching 240°C, add the reactor to hydrolyze for 240min to obtain resorcinol hydrolyzate, and obtain 276g of resorcinol (reduced pure) through extraction and rectification. The purity of resorcinol product (HPLC) is 99.7%. The molar utilization rate is 93.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com