Fingerprint map and high performance liquid chromatography identification method for Xuezhitong capsule
A high-performance liquid chromatography and fingerprint technology, which is applied in the identification of Xuezhitong Capsules by high-performance liquid chromatography and the establishment of Xuezhitong Capsules' fingerprints, can solve the problems of instability and errors of sulfur-containing compounds, and achieve a moderate ratio. , uniform distribution, good overall fingerprint effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
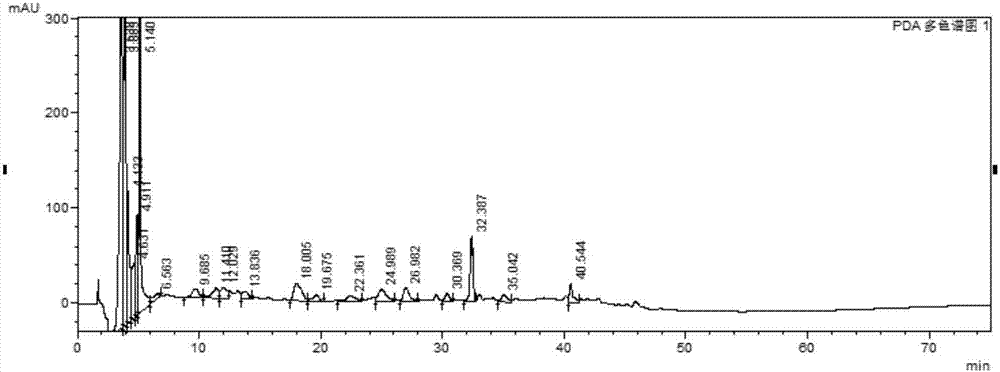
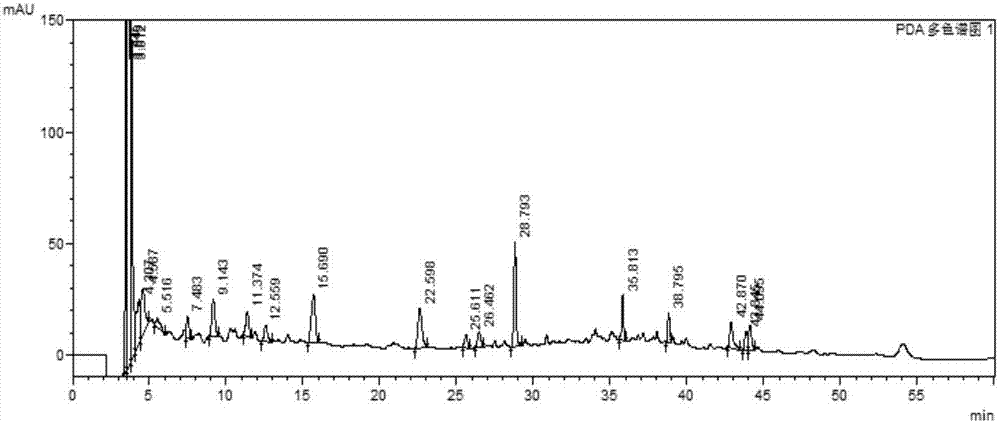
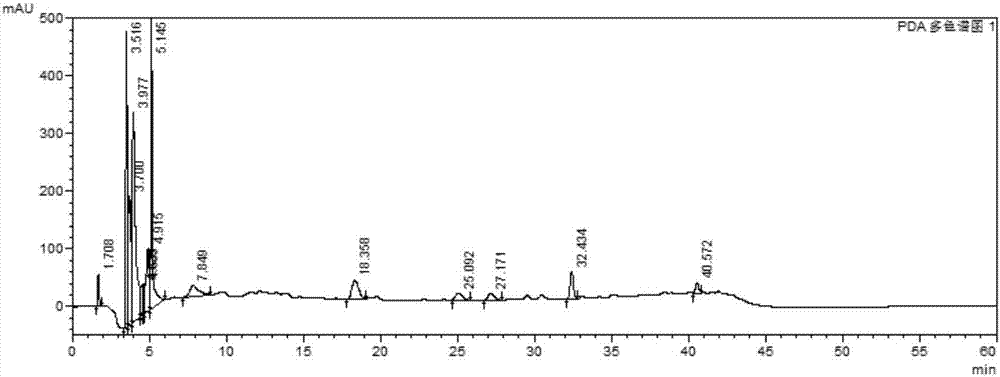
[0059] Example 1 Establishment of Xuezhitong Capsule Fingerprint
[0060] 1. Experimental materials
[0061] 1.1 Sample
[0062] 血滞通胶囊由吉林省东方制药有限公司提供,共20批,批号分别为:20120804、20120810、20120903、20120906、20120907、20130102、20130103、20130104、20130105、20130706、20130707、20130902、20140408、20140409、20140410 , 20150101, 20150502, 20150503, 20150603, 20150604. There are 4 batches of allium medicinal materials, the batch numbers are: YZ-31-150501, YZ-31-141101, YZ-31-140901, YZ-31-140801. 4 batches of intermediates, the batch numbers are: 20150514, 20150601, 20150602, 20150603.
[0063] 1.2 Instruments and reagents
[0064] 1.2.1 Instruments
[0065] Shimadzu LC-20AT high performance liquid chromatography, LC-solution workstation (Shimadzu, Japan). Chinese medicine fingerprint similarity evaluation system software version 2.0; Agilent 1200Series high performance liquid chromatograph, Agilent Chem Station chromatographic workstation (Agilent, USA). KQ-200 Ultrasonic Cleaner (Kunshan Shum...
Embodiment 2
[0142] The high-performance liquid chromatography identification of embodiment 2 Xuezhitong Capsules
[0143] 1, experimental material is the same as embodiment 1 fingerprint spectrum research.
[0144] 2. The preparation of the test solution is the same as the fingerprint research in Example 1.
[0145] 3. The chromatographic experiment conditions are the same as those in Example 1 for fingerprint research.
[0146] 4. The screening of the preparation conditions of the test product is the same as in Experimental Example 1.
[0147] 5. Methodological validation
[0148] 5.1 Precision test: the same as in Example 1 for fingerprint research.
[0149] 5.2. Stability test: the same as in Example 1 for fingerprint research.
[0150] 5.3 Repeatability test: the same as in Example 1 for the fingerprint study.
[0151] 5.4 Specificity test
[0152] The prescription of Xuezhitong Capsules only contains the medicinal material Allium scallions, and the others are auxiliary material...
experiment example 1
[0158] Experimental Example 1 Screening of Preparation Conditions of Test Articles in the Establishment of Xuezhitong Capsules Fingerprint
[0159] 1. Selection of extraction solvent
[0160] The present invention has selected 30% methanol, 50% methanol, methyl alcohol, 50% ethanol and ethanol (both volume percent) to extract Xuezhitong capsules, contrast HPLC chromatographic peaks (other parameters and chromatographic experiment conditions for test solution preparation) All with embodiment 1). the result shows( Figure 28 ), 50% methanol as the extraction solvent has the largest chromatographic peak area, the best extraction efficiency, and the best overall appearance. Therefore, the present invention selects 50% methanol as the extraction solvent of Xuezhitong capsules.
[0161] 2. Selection of extraction method
[0162] The present invention respectively ultrasonically extracts once for 30 minutes, ultrasonically extracts twice for 30 minutes (power 250W, frequency 40KH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com