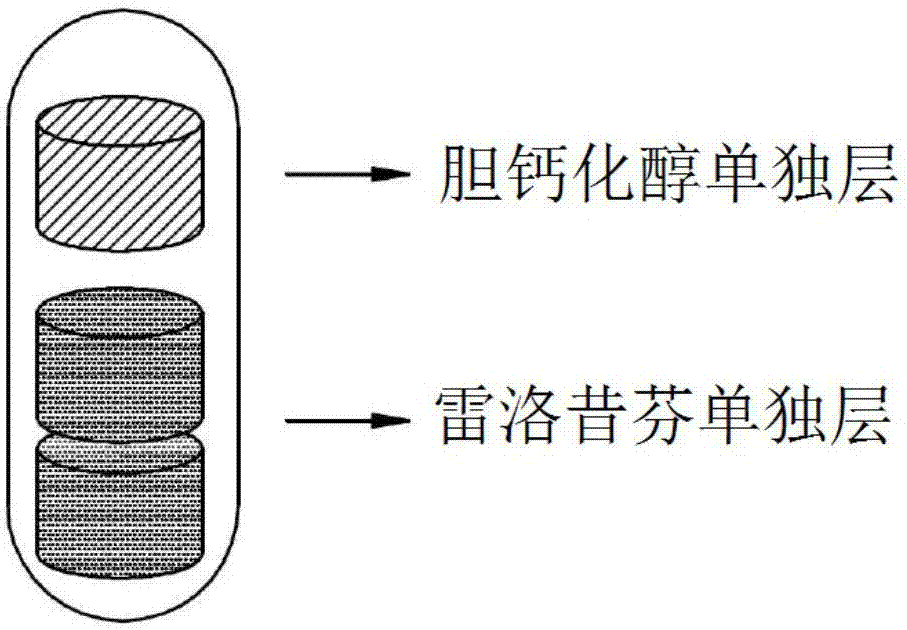
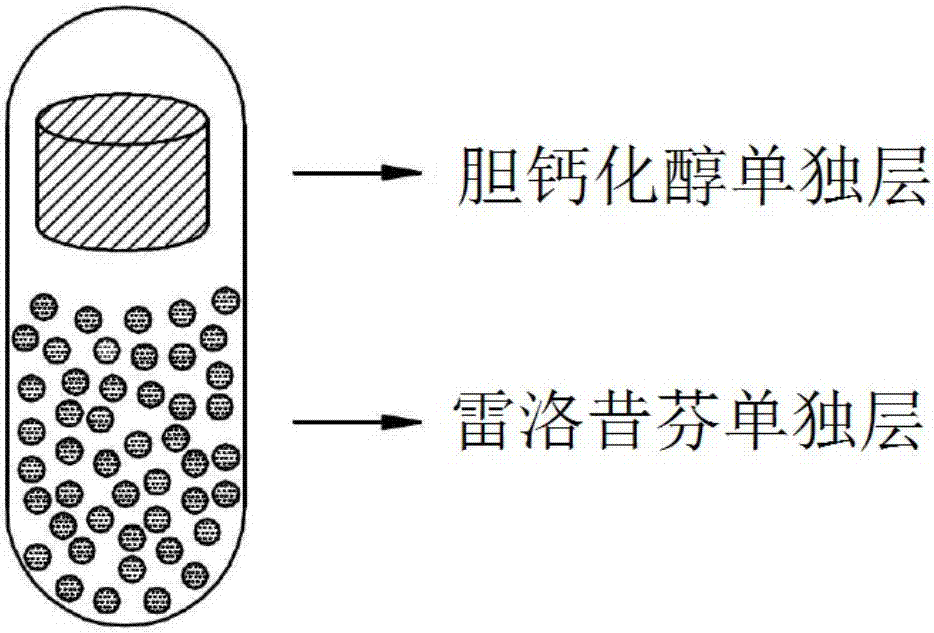
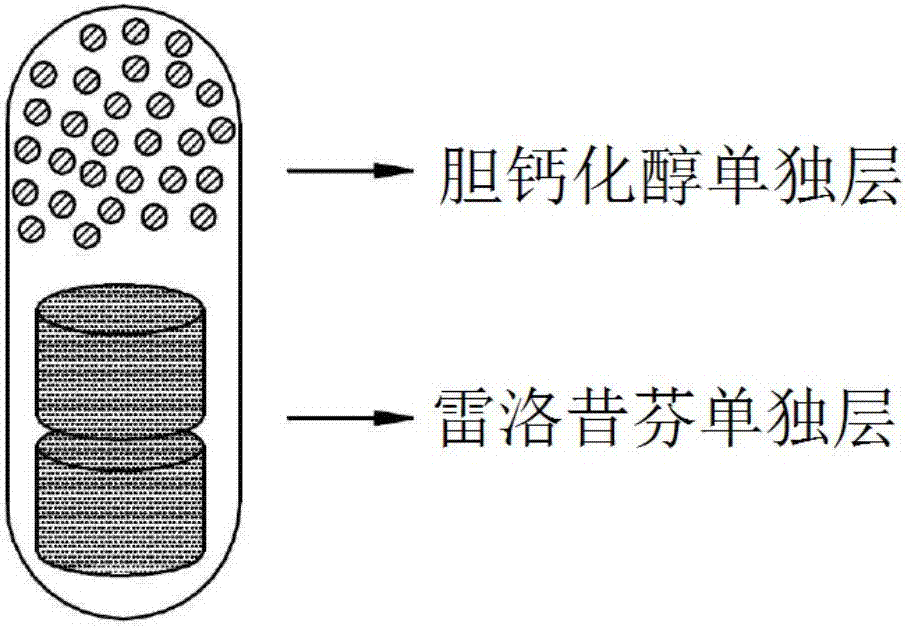
Composite capsules comprising raloxifene, and vitamin D or its derivatives
A technology of compound capsules and vitamins, applied in the directions of capsule delivery, microcapsules, drug combination, etc., can solve the problems of poor biochemical stability and inability to guarantee stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: Preparation I of compound preparation
[0068] Povidone K30 (Povidone K30) and polysorbate 80 (Polysorbate80) shown in Table 1 were dissolved in ethanol and distilled water to obtain a binder solution. The other ingredients of Table 1 were mixed, then wet granulated with a binder solution, then sieved through a sieve with a mesh size of 30, and dried to obtain dry granules. The resulting dried raloxifene granules were compressed with a round punch having a diameter of about 5.5 mm to prepare raloxifene tablets. Next, Opadry White was dissolved in distilled water and ethanol to prepare a coating solution, and then the raloxifene tablet was coated with the coating solution.
[0069] The ingredients of the cholecalciferol-containing layer of Table 2 were mixed together and then compressed to obtain cholecalciferol tablets. Opadry White and Blue No. 2 colorants were dissolved in distilled water and ethanol to obtain a coating solution, and then cholecalcif...
Embodiment 2
[0076] Embodiment 2: the preparation II of compound preparation
[0077] A composite capsule comprising 60 mg of raloxifene and 800 IU of cholecalciferol was prepared in the same manner as in Example 1, except that a hard capsule comprising hypromellose as a main material was used.
Embodiment 3
[0078] Embodiment 3: the preparation III of compound preparation
[0079] A composite capsule comprising 60 mg of raloxifene and 800 IU of cholecalciferol was prepared in the same manner as in Example 1, except that a hard capsule comprising pullulan as a main material was used.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com