Application of zoledronic acid compound in preparation of drugs for treating cancers
A technology of zoledronic acid and compounds, applied in the field of medicinal chemistry, can solve the problems of no cancer cell inhibitory effect, difficult to predict the drug effect of cancer cells, no proposed anti-cancer effect, etc., and achieve the effect of strong cytotoxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
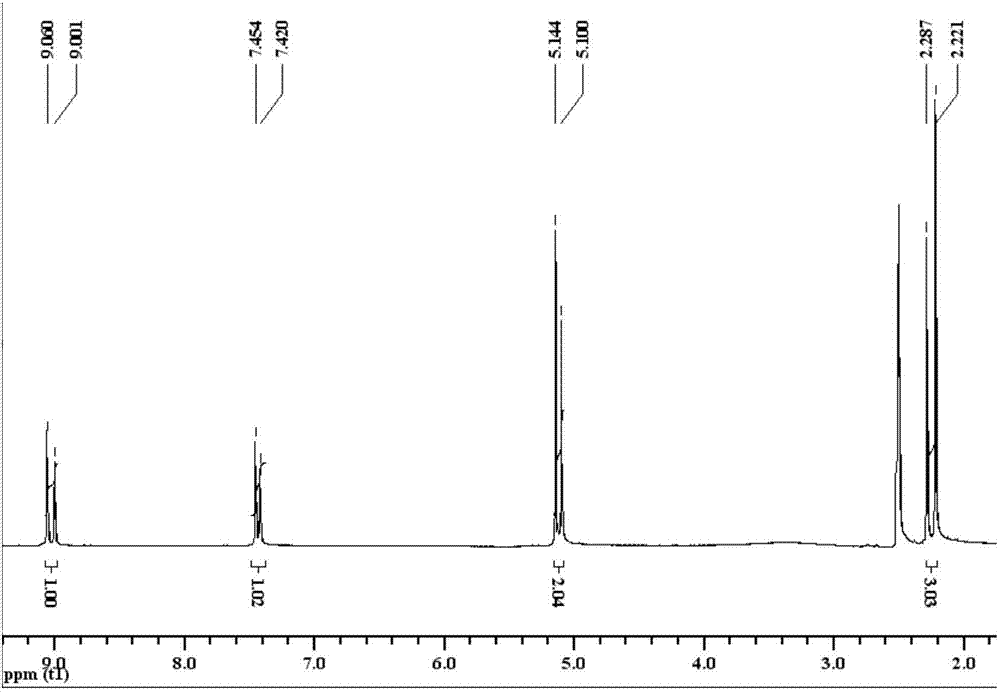
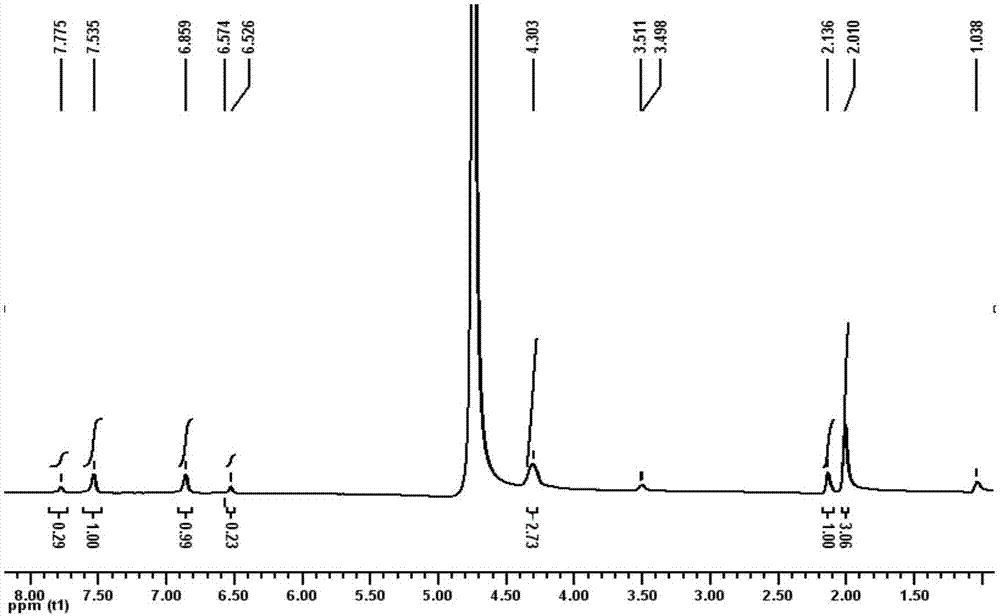
[0044] The present embodiment provides a kind of method for preparing the compound shown in formula (I), comprises the following steps:
[0045]
[0046] The synthetic route of compound (I) is:
[0047]
[0048] Synthesis of the compound 2-(4-methyl-1H-imidazolyl) ethyl acetate shown in Q1, formula (A-2)
[0049] In a 150mL three-necked flask, add 8.2g (0.1mol) of the compound 4-methylimidazole shown in formula (A-1), 8.4g (0.15mol) of KOH, KOH 2 CO 3 13.8g (0.1mol), tetrabutylammonium bromide 0.7g (2mmol) and CH 2 Cl 2 75mL, after stirring at room temperature for 0.5h, 11.2mL (0.1mol) of ethyl bromoacetate was slowly added dropwise to it, refluxed for 8h and filtered, and the filter cake was washed with CH 2 Cl 2 40mL was washed twice, the filtrate was washed with 150mL saturated NaCl solution, the layers were separated, and the organic phase was washed with anhydrous NaCl 2 SO 4 After drying, the solvent was distilled off to obtain 10.6 g of a brown viscous liq...
Embodiment 2
[0055] This example provides an anticancer drug, which uses the compound represented by the formula (I) prepared in Example 1 as an active ingredient.
[0056] Further, the anticancer drug is a clinically acceptable preparation made by selectively adding conventional excipients according to a conventional process, using the compound represented by the formula (I) as an active ingredient, and the preparation includes a tablet , capsules, granules, syrups, powders, pills, tinctures, liquors, decoctions, freeze-dried powders, lozenges or mixtures.
experiment example
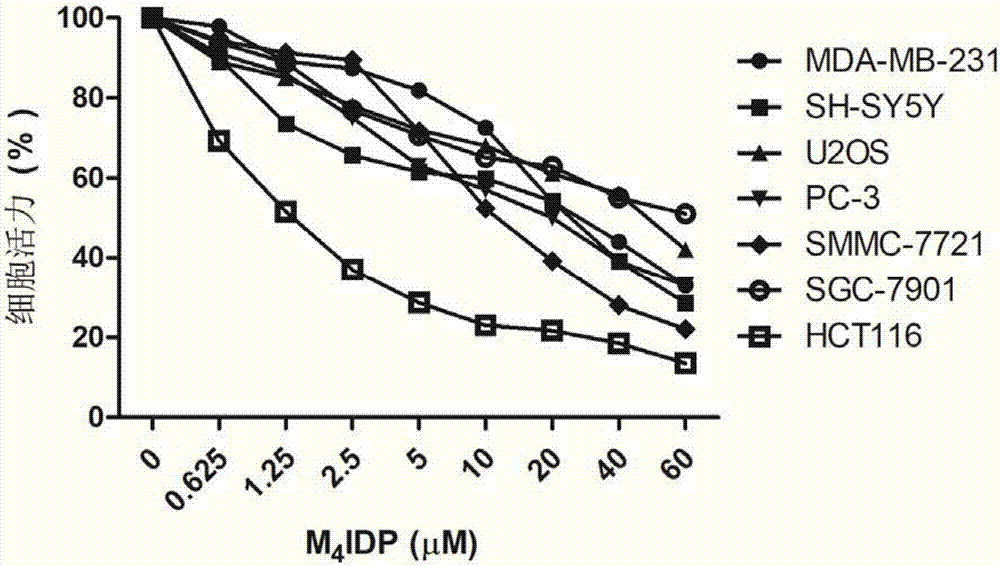
[0058] Compound 1-hydroxyl-2-(4-methyl-1H-imidazolyl)-ethane-1 shown in formula (I) of the present invention, 1-bisphosphonic acid (M 4 IDP) inhibitory effect on cancer cells
[0059] The experimental materials involved in the following experimental examples are as follows:
[0060] Fetal bovine serum (FBS), antibiotics, and DMEM were purchased from Gibco in the United States;
[0061] Hoechst33258, monodansylcadaverine (MDC), MTT, and DMSO were purchased from Sigma, USA;
[0062] Annexin V / PI staining kit was purchased from Roche;
[0063]CCK-8 kit, crystal violet, cell cycle analysis kit, reactive oxygen species (ROS) analysis kit, RIPA lysate, adenovirus expressing mCherry-GFP-LC3B (Ad-mCherry-GFP-LC3B), and p27, Cyclin D1, PARP, active-caspase3, CHOP, Bip, LC3B, PTEN, phospho-GSK-3β(Ser9), GSK-3β, Bad and other antibodies were purchased from Biyuntian Company;
[0064] Antibodies such as phospho-Rb (Ser780), ERO1L, p62, ATG5, and phospho-PDK1 (Ser241) were purchased fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com