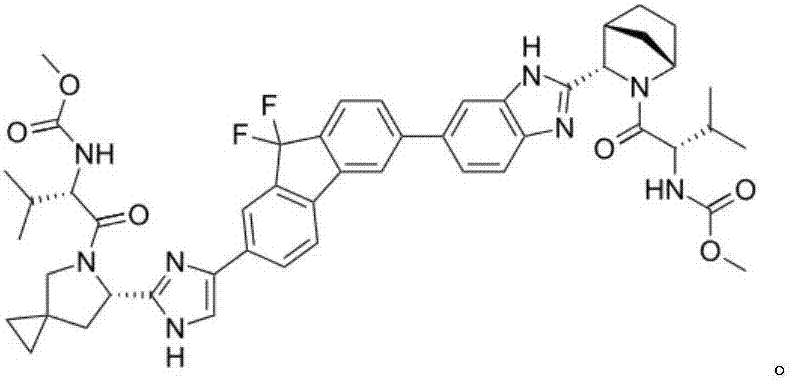
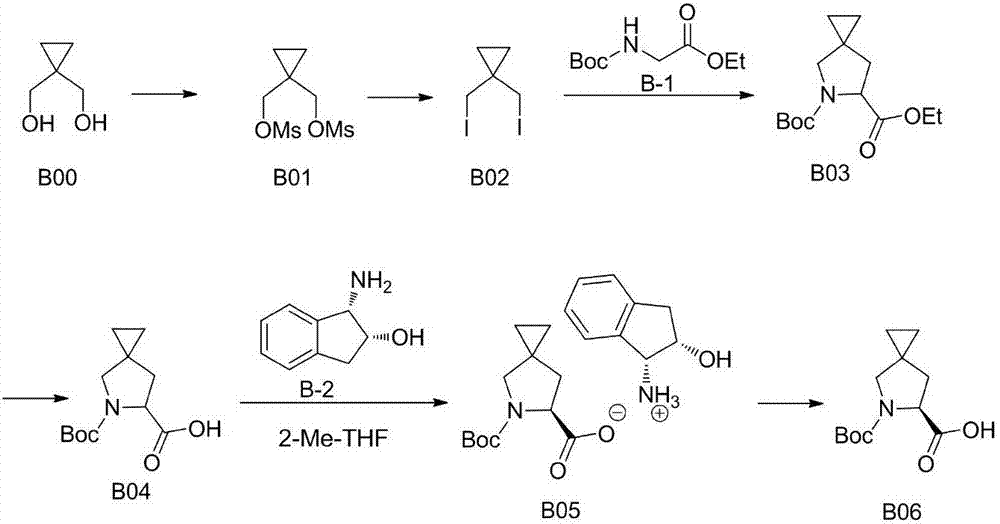
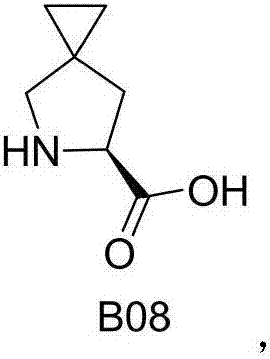
Improved method for preparing ledipasvir optical intermediate
A technology for ledipasvir and intermediates, applied in the field of improved preparation of ledipasvir optical intermediates, which can solve the problems of high cost, low yield, waste of production resources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] In some embodiments, the preparation method of B06 of the present invention comprises the following steps:
[0038] a) Add 5-azaspiro[2.4]heptane-6-carboxylic acid, benzaldehyde, D-tartaric acid and n-butyric acid into the reaction vessel, control the temperature at 35° C. to 100° C. for 5 hours to 25 hours, and drop to At room temperature, the solid was separated, and the obtained solid was dried in vacuum at 60° C. to obtain (S)-5-azaspiro[2.4]heptane-6-carboxylic acid or a salt thereof;
[0039] b) Add (S)-5-azaspiro[2.4]heptane-6-carboxylic acid D-tartrate, water and dichloromethane into the reaction vessel, stir at room temperature, adjust the pH to 1~ with dilute hydrochloric acid 4. After liquid separation, add the water phase to another reaction vessel, then adjust the pH value to 9~14 with aqueous sodium hydroxide solution, cool down to -10°C~30°C, then add di-tert-butyl dicarbonate, and stir the reaction After 1 hour, the temperature was raised to 20°C-70°C f...
specific Embodiment approach
[0043] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0044] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0045] L refers to liters, g refers to grams, h refers to hours, N refers to moles / liter, 1H NMR refers to nuclear magnetic hydrogen spectrum, HPLC refers to high performance liquid chromatography, ℃ refers to degrees Celsius, D 2 O refers to deuterated water.
Embodiment 1
[0047]
[0048] Add 100g of 5-(tert-butoxycarbonyl)-5-azaspiro[2.4]heptane-6-carboxylic acid and 1L of water into a 2L reaction flask equipped with a magnet, start stirring, heat up to reflux, and reflux After stirring and reacting for 5 hours, the water was evaporated to dryness, and the obtained solid was vacuum-dried overnight to obtain 57.32 g of white solid, with a yield of about 98%. 1 HNMR (D 2 O) δ = 4.23(m,1H); 3.23(m,2H); 2.27(m,1H); 1.98(m,1H); 0.67(m,4H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com