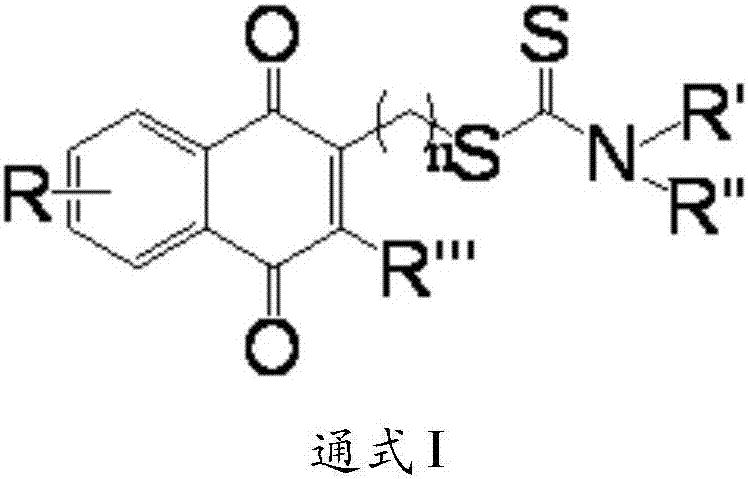
Aminodithioformate compounds, preparation method therefor and use of aminodithioformate compounds in preparation of antitumor drugs
A technology of aminodithiocarboxylate and ester compounds, which can be used in antitumor drugs, active ingredients of heterocyclic compounds, drug combinations, etc., can solve the problem of unsatisfactory pyruvate kinase subtype selectivity, low activity, and unclear tumors question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
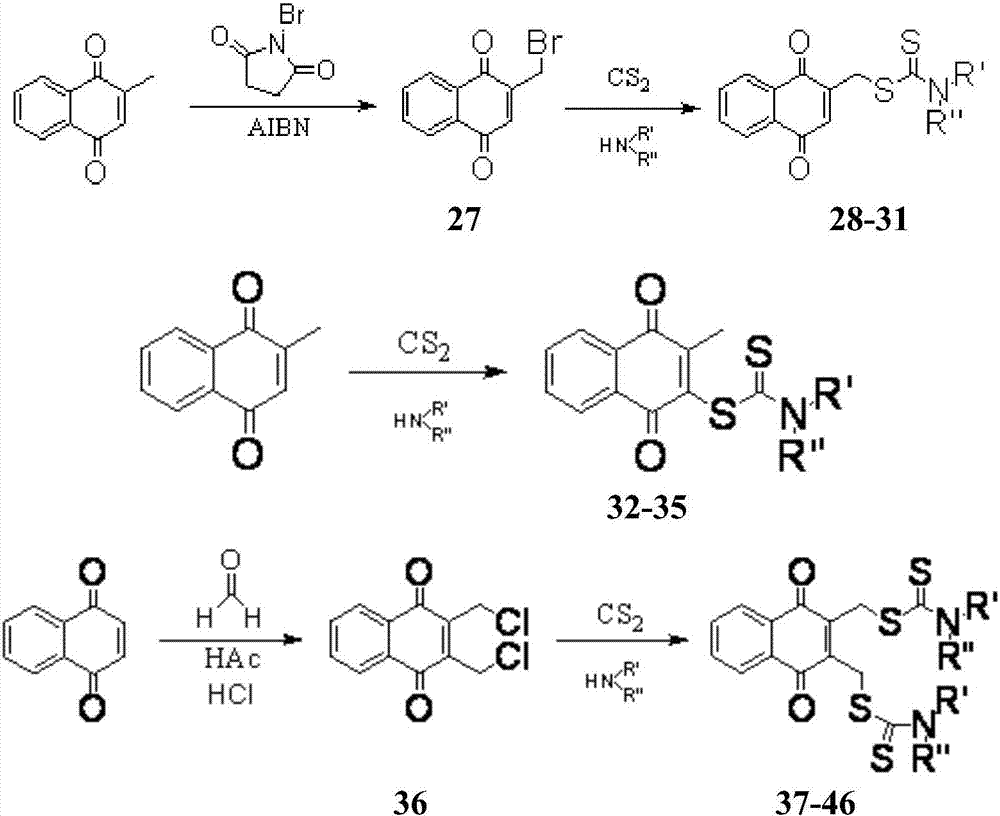
Method used
Image
Examples
Embodiment 1
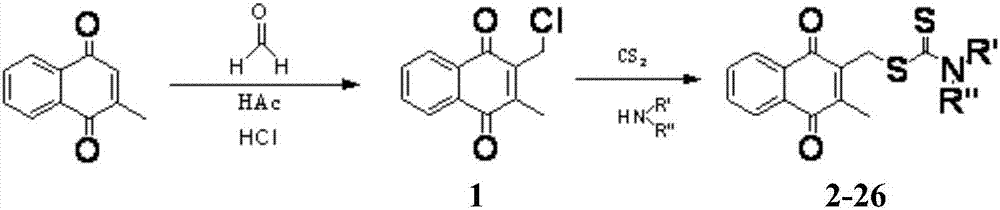
[0088] Example 1: Preparation of 2-chloromethyl-3-methyl-1,4-naphthoquinone (1)
[0089] Dissolve menadione (1g, 5.81mmol) in glacial acetic acid (10ml), add 36% formaldehyde aqueous solution (3ml), add hydrogen chloride gas under ice bath, stir for 30min, until the solution turns red, stir at room temperature React overnight. The solution turned dark brown, the reaction solution was poured into ice water, extracted with ethyl acetate, washed with saturated brine, dried with anhydrous sodium sulfate, and separated and purified with a silica gel column (eluted with petroleum ether / ethyl acetate) to obtain a yellow solid 1. 960mg, yield 75.0%, 1 H NMR(400MHz, CDCl 3 )δ8.13-8.18(m,2H,ArH),7.76-7.78(m,2H,ArH),4.64(s,2H,CH 2 S),2.35(s,3H,C=CCH 3 ).
Embodiment 2
[0090] Example 2: Preparation of 3-methyl-1,4-diox-1,4-dihydronaphthalene-2-methyl diethylaminodithioformic acid (2)
[0091] Dissolve diethylamine (123μL, 1.2mmol) in acetonitrile (10ml), add carbon disulfide (72μL, 1.2mmol), stir the reaction at room temperature for 30min, add 2-chloromethyl-3-methyl-1,4- Naphthoquinone (220mg, 1mmol), stir the reaction at room temperature, TLC detects the disappearance of the raw material point, terminate the reaction, spin down the solvent under reduced pressure, add a small amount of water, extract with ethyl acetate, combine the ester layers, wash with saturated brine, and anhydrous sodium sulfate Dry, separate and purify with silica gel column (petroleum ether / ethyl acetate elution) to obtain a yellow solid 2,298mg, yield 89.6%, mp 155-156°C. 1 H NMR(400MHz, CDCl 3 )δ8.11-8.14(m,2H,ArH),7.73-7.74(m,2H,ArH),4.65(s,2H,CH 2 S),4.06(q,2H,NCH 2 ),3.73(q,2H,NCH 2 ),2.37(s,3H,C=CCH 3 ),1.30(t,6H,2CH 2 CH 3 ). 13 C NMR(100MHz, CDCl 3 )δ194.49,184.8...
Embodiment 3
[0092] Example 3: Preparation of 3-methyl-1,4-diox-1,4-dihydronaphthalene-2-methyl dipropylaminodithioformic acid (3)
[0093] Using the synthetic method of compound 2 above, using 2-chloromethyl-3-methyl-1,4-naphthoquinone and dipropylamine as the reactants to obtain a yellow solid 3 with a yield of 86.0%, mp 80-81°C. 1 H NMR(400MHz, CDCl 3 )δ8.10-8.14(m,2H,ArH),7.72-7.74(m,2H,ArH),4.63(s,2H,CH 2 S),3.93(t,2H,NCH 2 ),3.61(t,2H,NCH 2 ),2.35(s,3H,C=CCH 3 ),1.70-1.82(m,4H,2CH 2 CH 3 ),0.92-0.98(m,6H,2CH 2 CH 3 ). 13 C NMR(100MHz, CDCl 3 )δ195.02,184.79,183.89,146.48,141.64,133.62,132.19,131.93,126.49,57.25,54.43,33.92,20.70,19.62,13.66,11.18.HR-MS(ESI + )m / z:362.1249[M+H] + .Found:362.1244[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com