Acellular cornea stroma lens and preparation method thereof
A corneal stroma and decellularization technology, applied in the field of tissue engineering, can solve the problems of unstable performance, poor transparency, and low safety of corneal lenses, and achieve the effects of improving mechanical strength, maintaining transparency, and avoiding immune rejection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
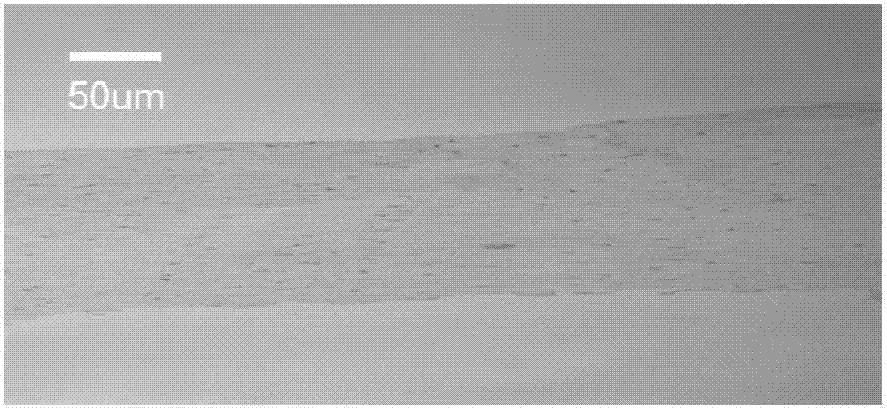
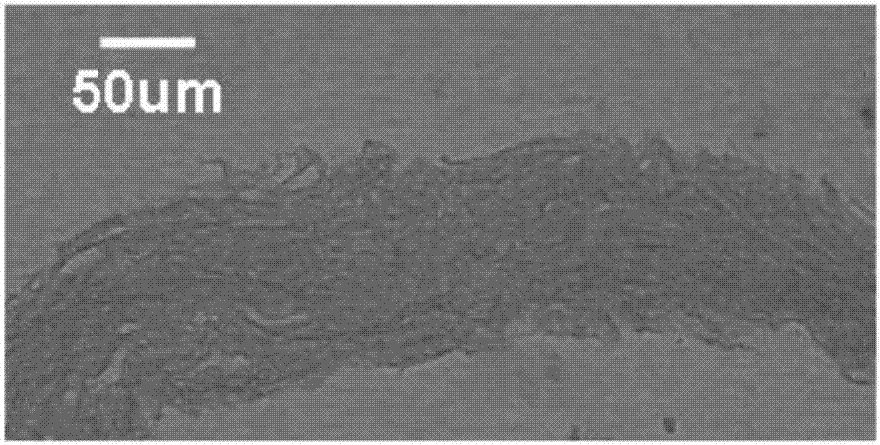
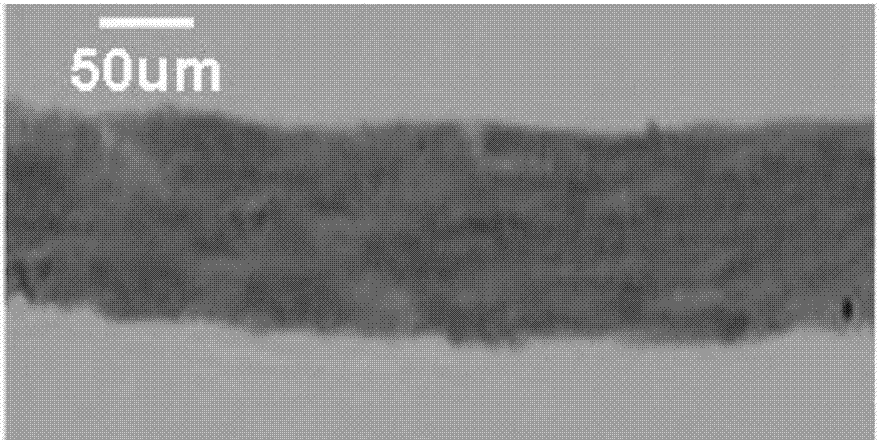
Image
Examples
preparation example Construction
[0033] The invention provides a method for preparing a decellularized corneal stroma lens.
[0034] Wherein, the 0.1%-3% TritonX-100 involved in the method of cell lysis can be, for example, but not limited to 0.1% TritonX-100, 0.3% TritonX-100, 0.5% TritonX-100, 0.8% TritonX-100, 1 %TritonX-100, 1.5% TritonX-100, 2% TritonX-100, 2.5% TritonX-100 or 3% TritonX-100.
[0035] Among them, the crosslinking agent involved in the crosslinking method can be, but not limited to, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide, or N-hydroxysulfosuccinimide , or genipin, or glutaraldehyde.
[0036] Wherein, the mass ratio of the cross-linking agent used in the cross-linking method to the corneal stromal lens is 1:1-1:15, and the mass ratio can be, but not limited to, 1:1, 1:2, 1 :3, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9, 1:10, 1:10, 1:11, 1:12, 1:13, 1:14 Or 1:15.
[0037] Among them, the concentration involved in the disinfection method is 0.01-0.1 mg / ml penicillin and 0.05-0.5 mg / ml streptom...
Embodiment 1
[0044] Embodiment 1 Preparation method of decellularized corneal stroma lens
[0045] Step (a), disinfection: Soak the corneal stromal lens with the accurate thickness of cells obtained by the full femtosecond laser technology in physiological saline containing 0.01 mg / ml penicillin and 0.1 mg / ml streptomycin 3h, then rinse with 0.9% saline;
[0046] Step (b), cell lysis: immerse in 0.9% normal saline containing 0.5% TritonX-100 for 24 hours, then rinse with 0.9% normal saline for 96 hours;
[0047] Step (c), cross-linking: Soak in 0.9% physiological saline containing a cross-linking agent, then rinse with 0.9% physiological saline for 24 hours, and the cross-linking agent is 1-(3-dimethylaminopropyl)-3-ethane Carbodiimide (EDC); the mass ratio of the amount of cross-linking agent to the corneal stroma lens processed through cell lysis is 1:5;
[0048] Step (d), sterilizing: irradiating the crosslinked corneal lens with gamma rays, and the irradiation dose is 25kGy;
[0049...
Embodiment 2
[0056] Embodiment 2 Preparation method of decellularized corneal stroma lens
[0057] Step (a), disinfection: Soak the corneal stromal lens with the accurate thickness of the cells obtained by the full femtosecond laser technology in physiological saline containing a concentration of 0.05 mg / ml penicillin and a concentration of 0.2 mg / ml streptomycin 2h, and then rinse with 0.9% saline;
[0058] Step (b), cell lysis: immerse in 0.9% normal saline containing 0.3% TritonX-100 for 48 hours, then rinse with 0.9% normal saline for 96 hours;
[0059] Step (c), cross-linking: Soak in 0.9% physiological saline containing cross-linking agent, then rinse with 0.9% physiological saline for 24h, cross-linking agent N-hydroxysulfosuccinimide (NHS); cross-linking agent The mass ratio of the usage amount to the corneal stroma lens processed by cell lysis is 1:9;
[0060] Step (d), sterilizing: irradiating the crosslinked corneal lens with gamma rays, and the irradiation dose is 25kGy;
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com