Patents
Literature
73 results about "Stroma of cornea" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
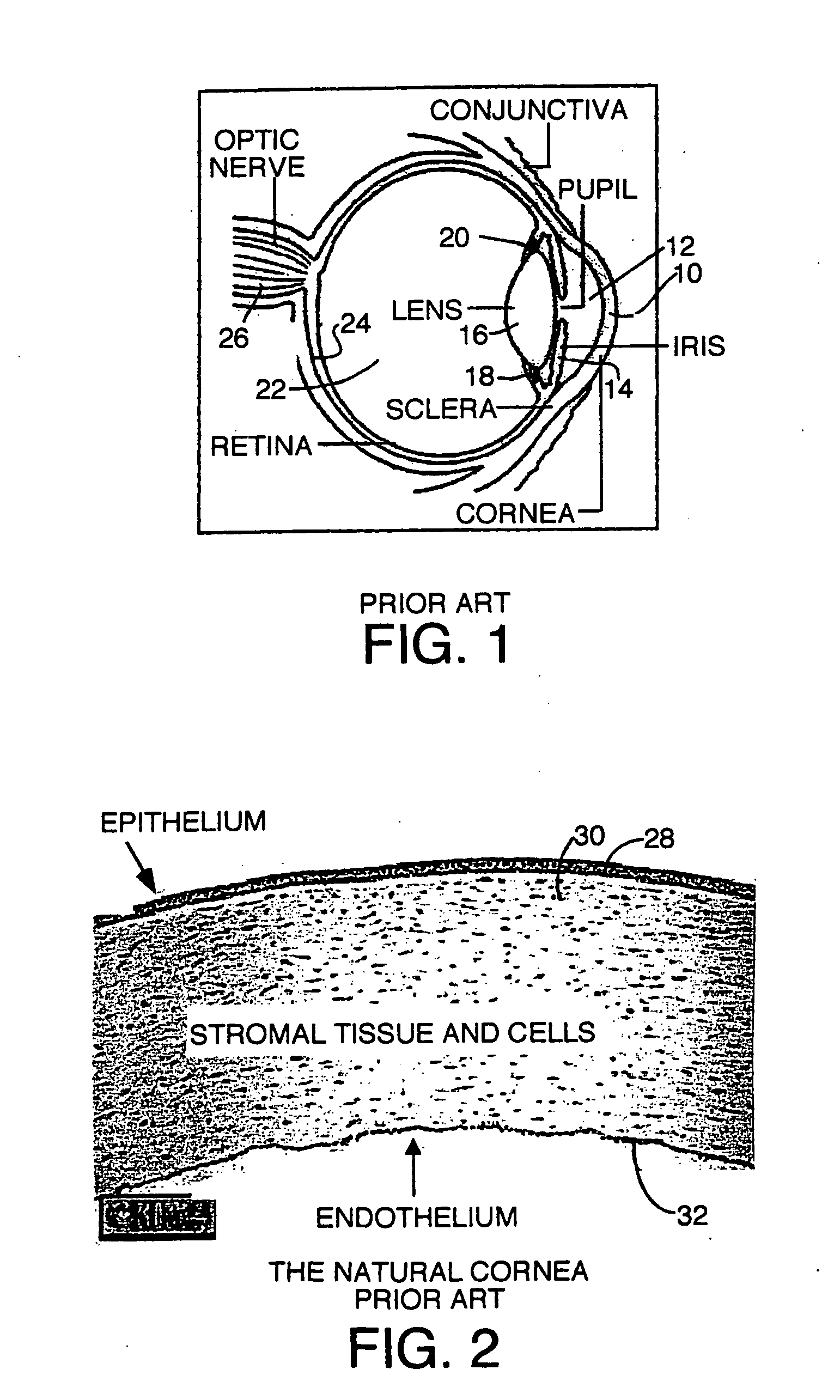
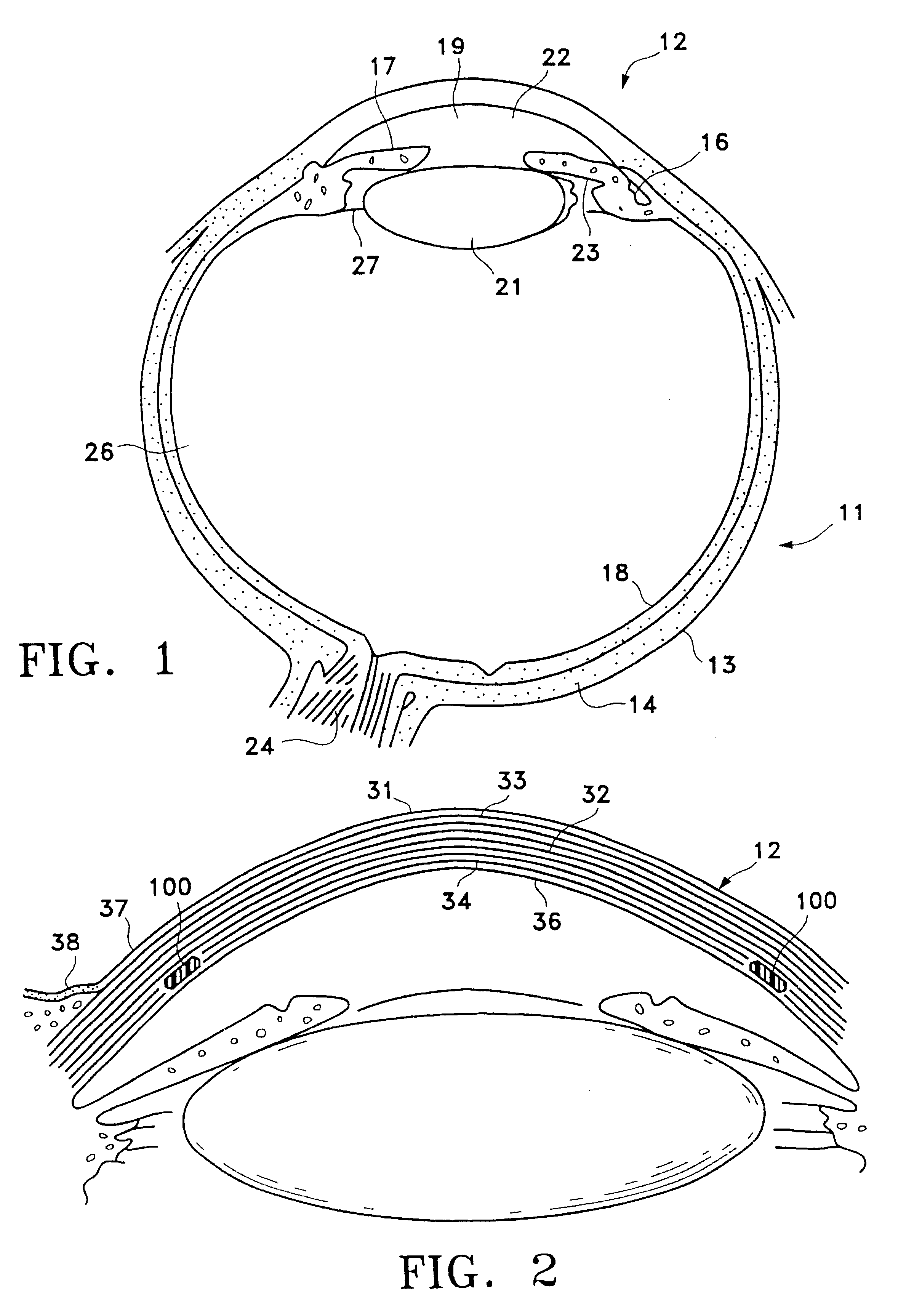
The substantia propria (or stroma of cornea) is fibrous, tough, unyielding, perfectly transparent and the thickest layer of the cornea of the eye. It lies below Bowman's membrane and above Descemet's membrane.
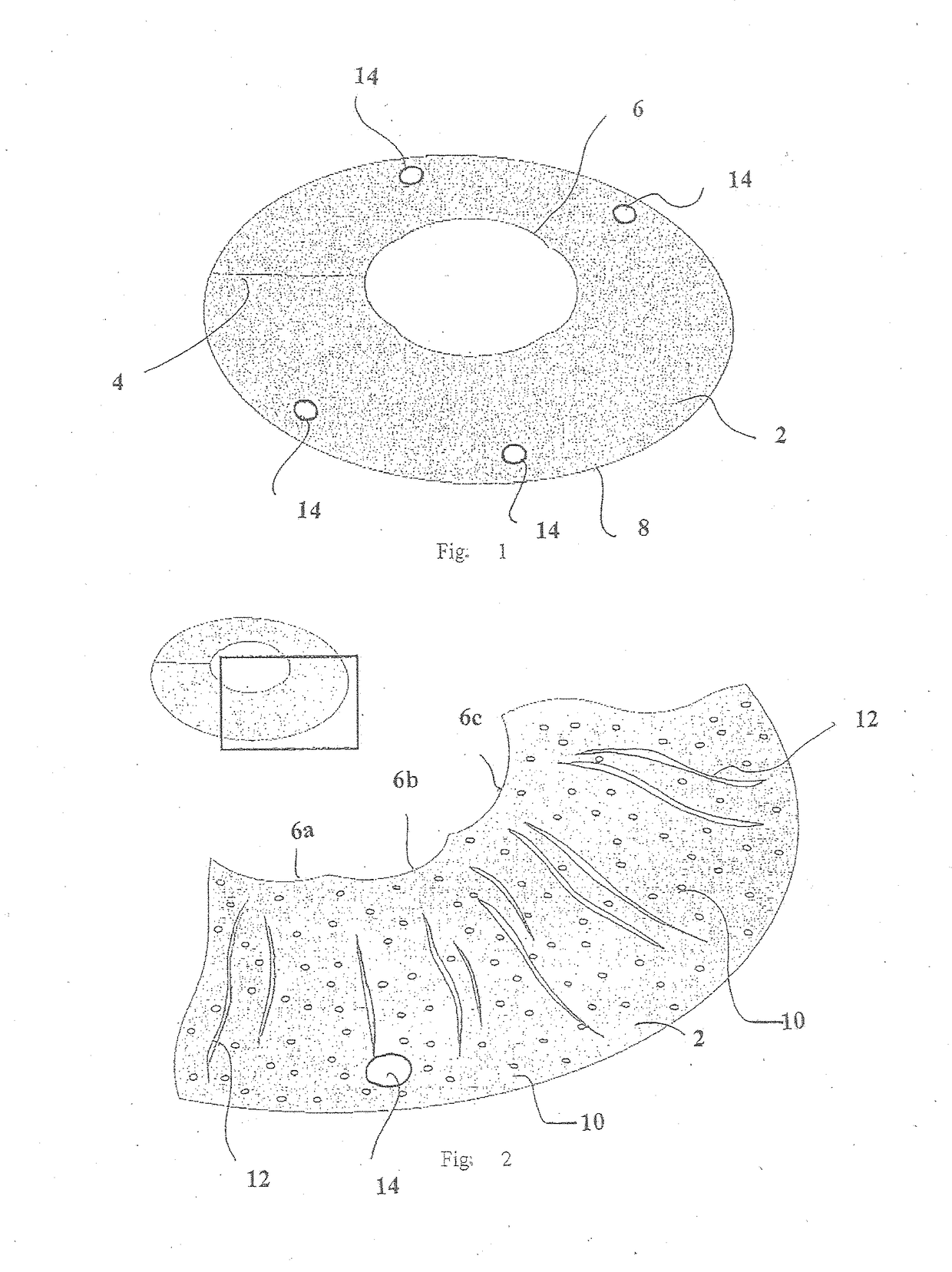
Exogenous cornea substrate without cells and its preparation method and use
The invention provides a cell-less heterogenetic cornea material and manufacturing method and use, the manufacturing method for the product is: acquires the fresh animals' cornea at first; eliminates the cell membrane of the cornea material and nucleic acid with chemical method and enzyme method; carries on dehydration process to the cell-less heterogenetic cornea material; stores for backup. The method eliminates the antigenicity of the heterogenetic cornea material through eliminating the cell elements in the cornea material. At the same time, it reserves the integrity of glue fiber and transparency of the cornea. The material can be applied to construction of manmade cornea or used as the medical material for cornea transplantation, and cornea refraction operation directly.
Owner:THE AFFILIATED SIR RUN RUN SHAW HOSPITAL OF SCHOOL OF MEDICINE ZHEJIANG UNIV
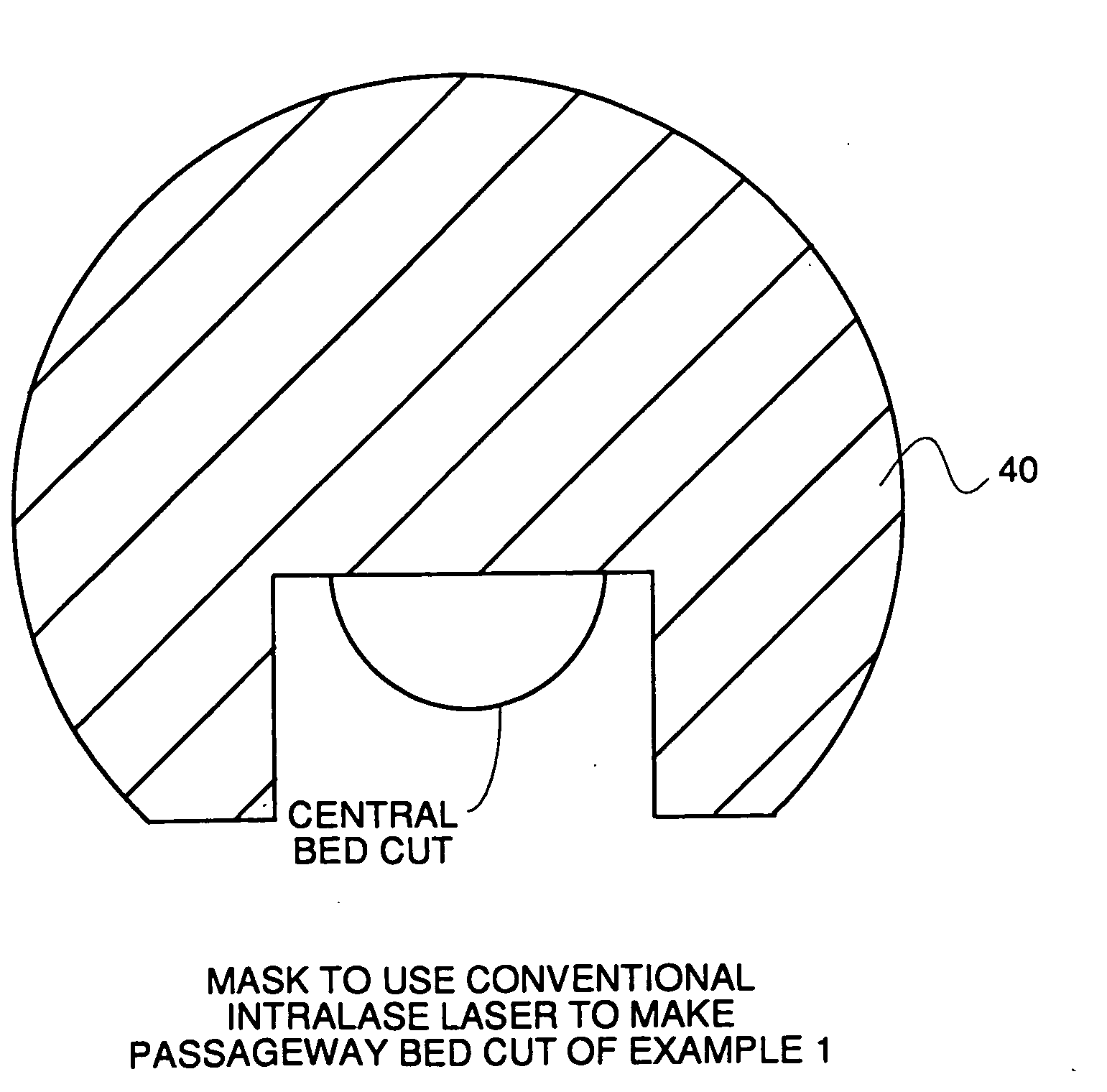
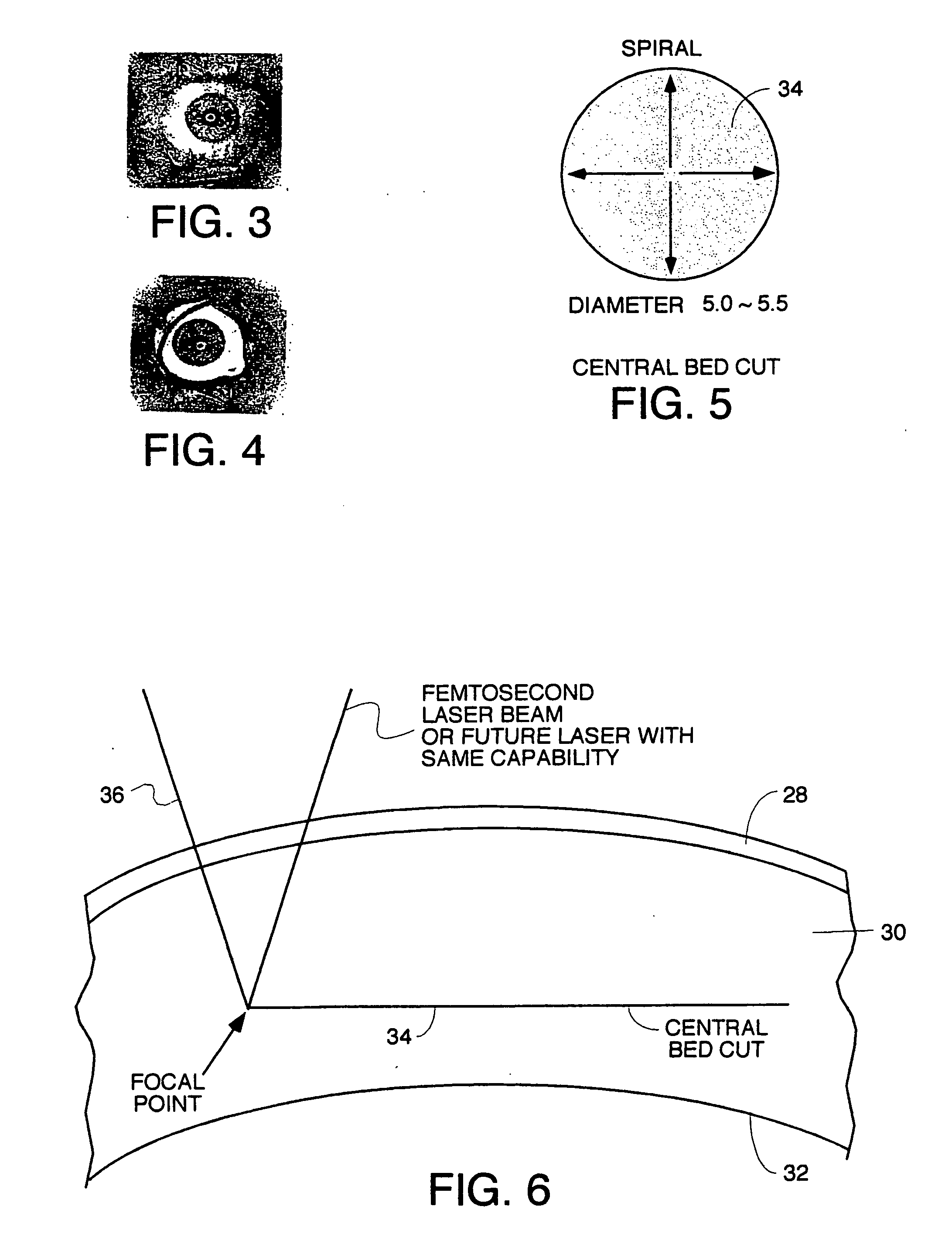
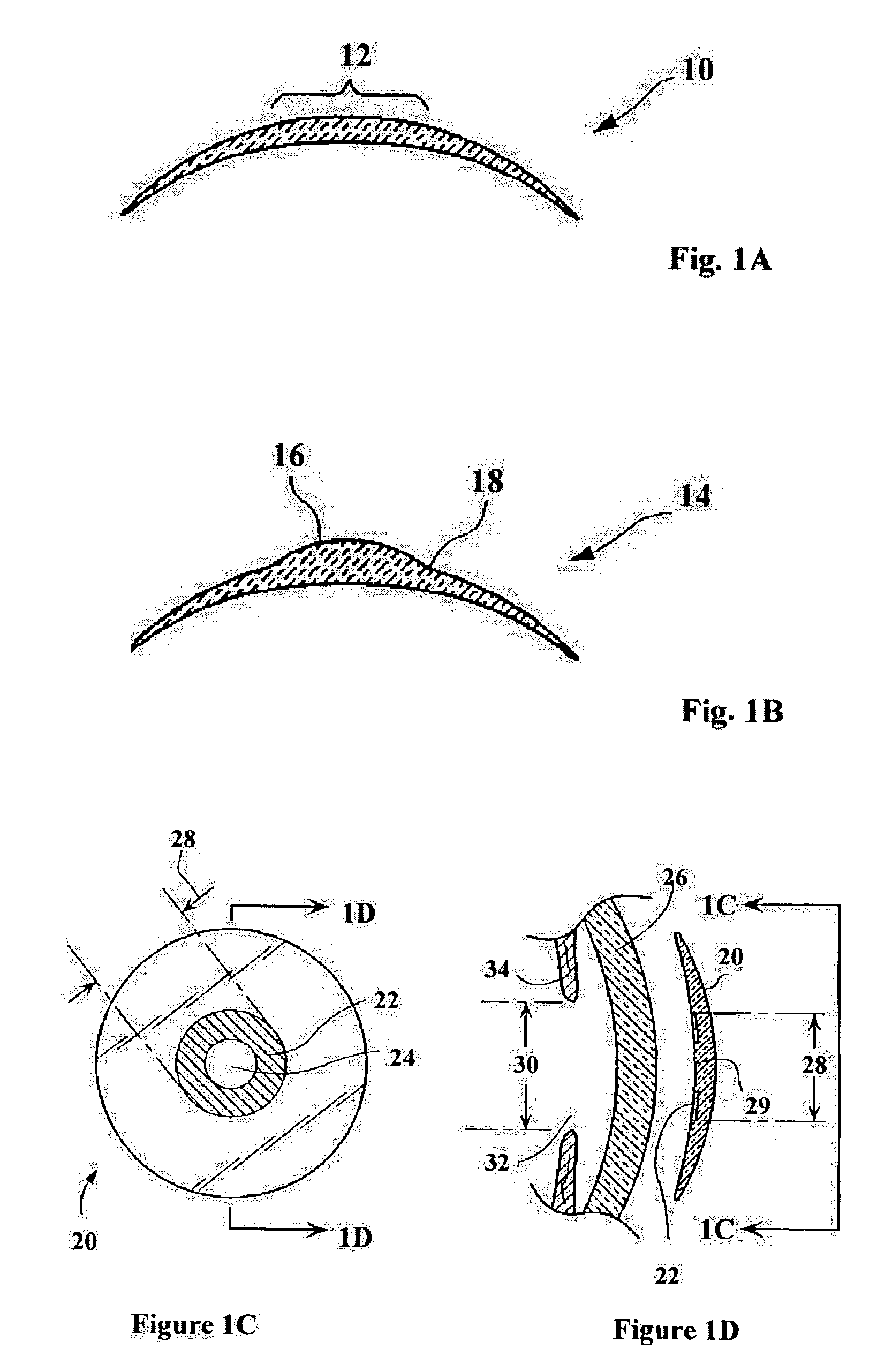
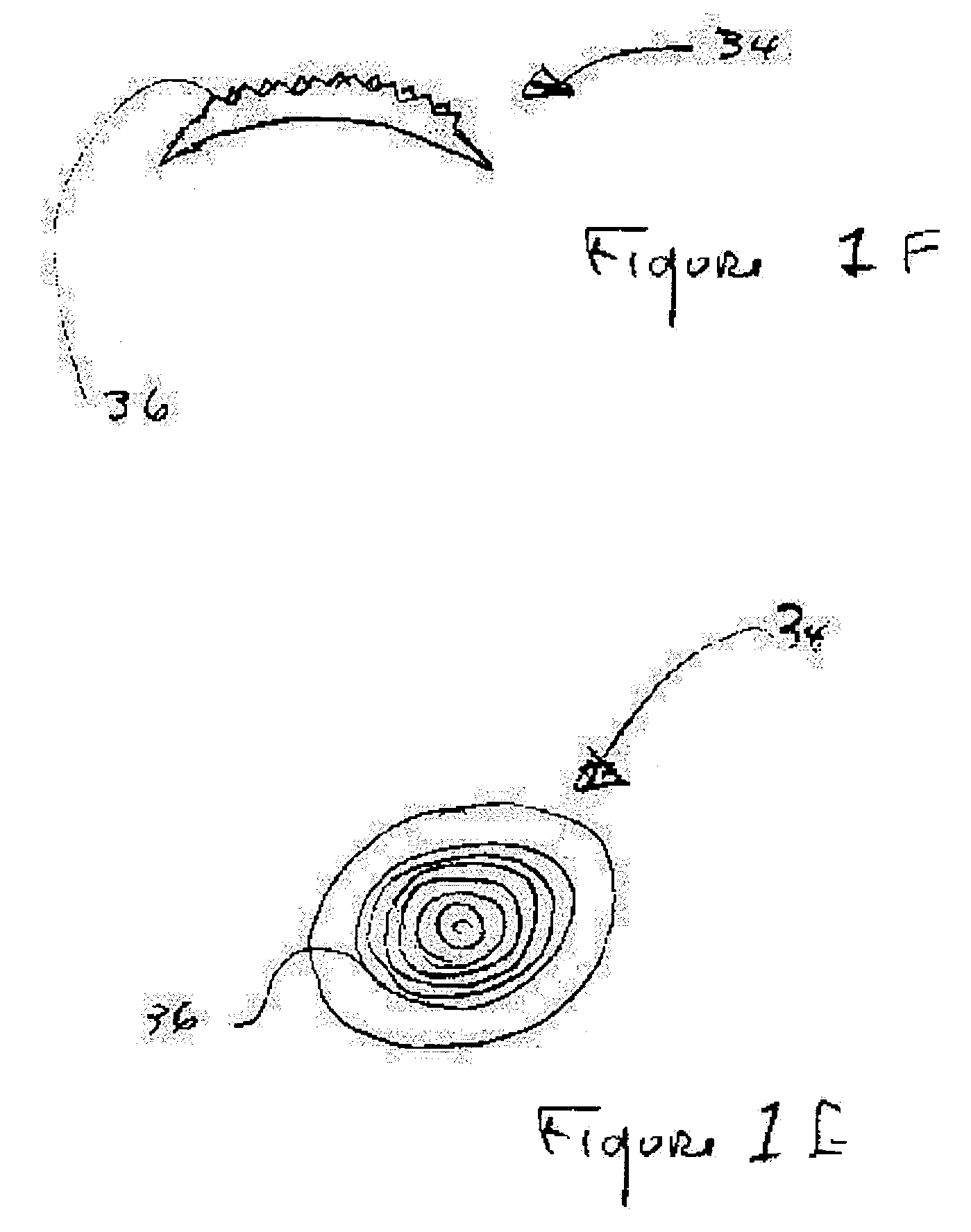
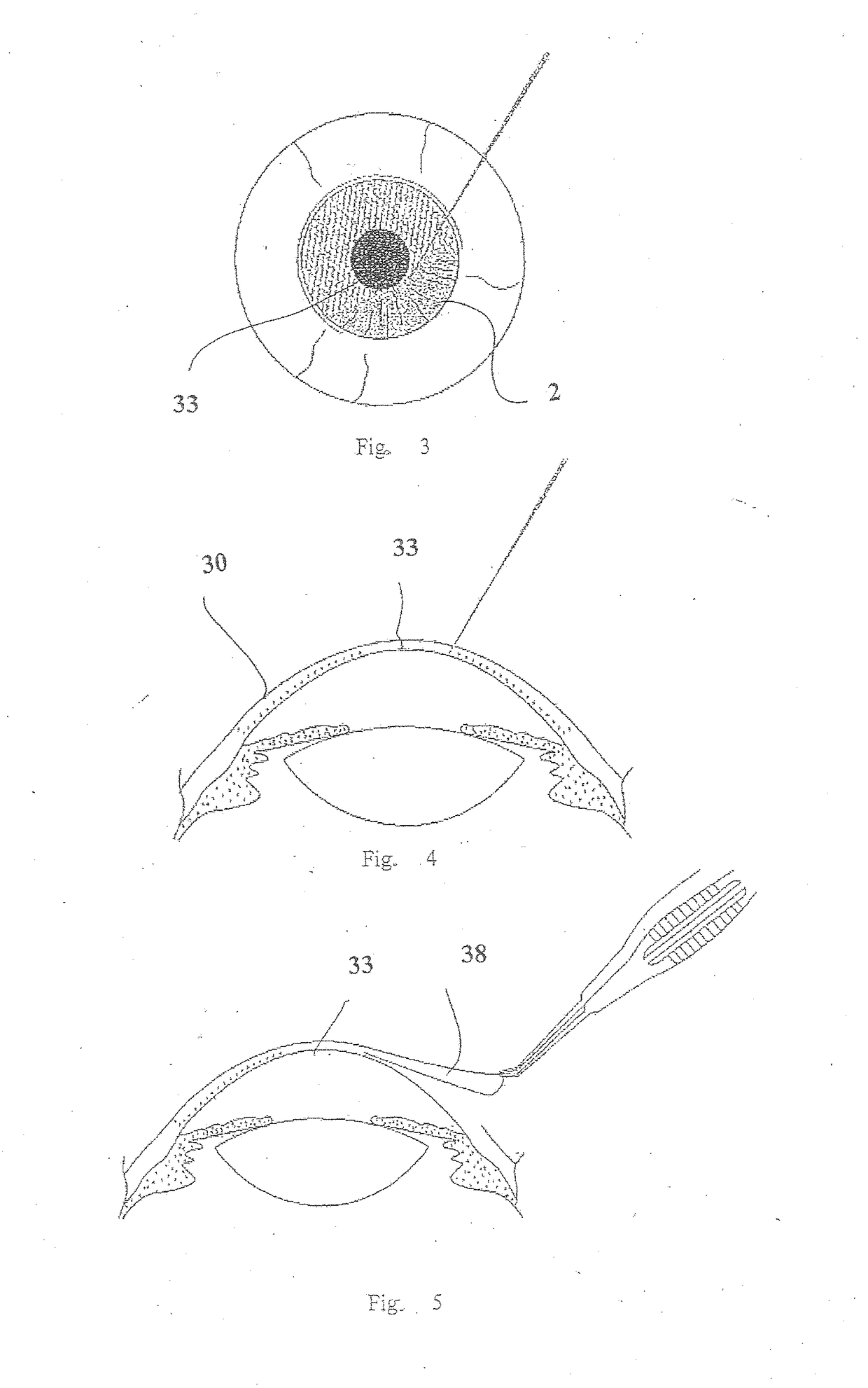
Surgical procedure and instrumentation for intrastromal implants of lens or strengthening materials
InactiveUS20070219542A1Improve permeabilityIncrease elasticityLaser surgerySurgical instrument detailsCorneal surfaceFemto second laser
A surgical procedure for implanting a lens or strengthening material in a cornea without making a flap as is done in Lasik procedures. A femtosecond laser or some future developed laser which has the same ability is modified to cut a pocket in the stroma layer of a cornea with no side cuts and only an opening at the surface of a cornea. Alternatively, a modified microkeratome shaped and sized to form the desired shape for a passageway bed cut which is cut to join a central bed cut. Alternatively, the passageway bed cut can be done using a femtosecond laser or some future developed laser which has the same ability. The passageway bed cut is smaller in dimension than a central bed cut of a pocket in which the lens will be implanted in procedures where an expandable lens or strengthening material is used. A modified microkeratome having a web, a central blade shaped to form the passageway bed cut and sled runner on either side of the blade and separated therefrom is also disclosed. A patient interface with a mask is also disclosed to allow a femtosecond laser or some future developed laser which has the same ability may also be used to form both the central bed cut and a passageway bed cut or only a single bed cut with an opening at the surface of the cornea to form the pocket is also disclosed.
Owner:YAHAGI TORU
Methods for producing epithelial flaps on the cornea and for placement of ocular devices and lenses beneath an epithelial flap or membrane, epithelial delaminating devices, and structures of epithelium and ocular devices and lenses
InactiveUS7497866B2Enhances delamination processSpeed up the processEye surgerySurgeryCorneal surfaceLens crystalline
These methods, devices, and structures are useful in the field of ophthalmology; the devices and methods relate variously to separating or lifting corneal epithelium from the eye preferably in a substantially continuous layer, placing a lens or other suitable ocular or medical device beneath the epithelial membrane, and to the resulting structures formed by those procedures. The de-epilthelialization devices generally utilize a non-cutting separator or dissector that is configured to separate the epithelium at a naturally occurring cleavage surface in the eye between the epithelium and the corneal stroma (Bowman's membrane), specifically separating in the region of the lamina lucida. The separator or dissector may have a structure that rolls or vibrates (or both) at that cleavage surface or interface during the dissection step. The separated epithelium may be lifted or peeled from the surface of the eye to form an epithelial flap or a pocket. The epithelium may then be replaced on the cornea after a refractive procedure or after placement of an ocular lens (or other subepithelial device) on the eye. The subepithelial device may comprise a wide variety of synthetic, natural, or composite polymeric materials. The step of replacing epithelial tissue upon the subepithelial device or upon the anterior corneal surface promotes epithelial healing.
Owner:TISSUE ENG REFRACTION
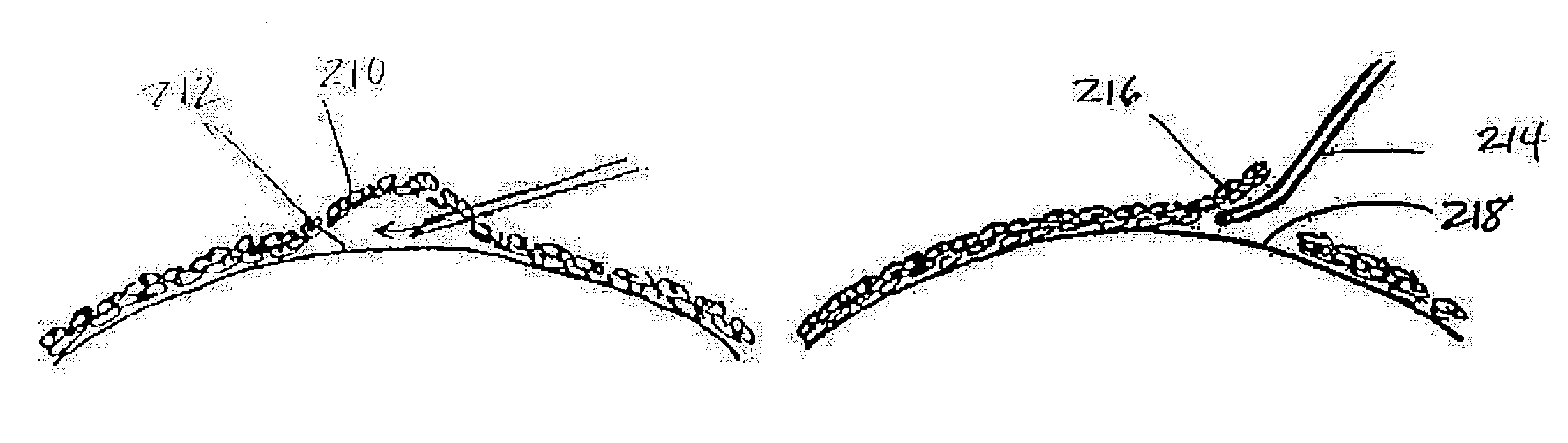
Device and method for inserting a biocompatible material into the corneal stroma
This invention is an improved method and kit for producing a desired channel or pathway in the interlamellar space in the corneal stroma for inserting a biocompatible material. The biocompatible polymer may be an intrastromal corneal ring (ICR). The method involves the use of clockwise and counter-clockwise dissectors, and optionally channel connectors and finish channel connectors. The kit contains clockwise and counter-clockwise dissectors and optionally channel connectors, finish channel connectors and probes.
Owner:ADDITION TECH INC
Ply tissue engineering corneal frame and manufacturing method and application thereof
ActiveCN101947144AThe production process is simple and reliableShorten the timeEye implantsDiseaseFiber
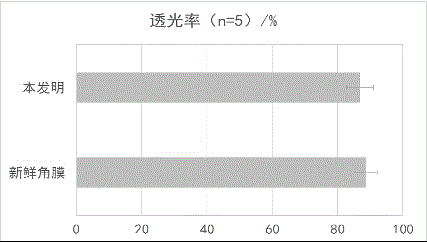
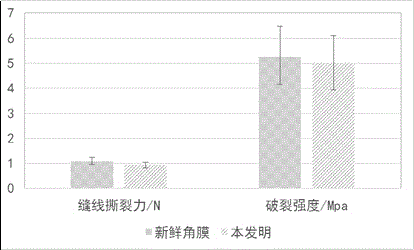
The invention discloses a ply tissue engineering corneal frame and a manufacturing method and an application thereof, relating to a ply tissue engineering corneal frame of the biomedicine. Compared with the existing materials, the invention provides a ply tissue engineering corneal frame and a manufacturing method and an application thereof, which has abundant resources, high transparency, good biocompatibility, thorough decellularization and strong safety, and the performance approaches to that of a fresh cornea, so that the ply tissue engineering corneal frame can be accepted by majority of patients and applied clinically for a long term. The ply tissue engineering corneal frame is an animal derived decellularized ply corneal stroma sheet and does not contain cellular constituents; collagenous fibers are tidily arranged, and gaps are regular; corneal light transmittance is 80-95%, and tensile strength is 2-5N / mm<2>. The ply tissue engineering corneal frame can be served as the substitute for various donor materials for corneal transplantation and can be used for treating a series of diseases of corneal trauma and chemical burn, corneal tumor and a series of hyperplastic diseases, a series of diseases of corneal neovascularisation and scar, corneal immune diseases, a series of diseases caused by corneal transplantation exclusive reaction and other keratopathy.
Owner:XIAMEN DAKAI BIOTECH CO LTD
Decellularization cornea preparation method
The invention relates to a decellularization cornea preparation method, which adopts steps of corneal epithelium layer removing, ultraviolet cross-linking, viral inactivation, decellularization treatment, gradient dehydration, radiation protection and sterilization. According to the present invention, the prepared decellularization cornea has characteristics of complete antigen removing, no excitation of host acquired immunity reaction, good biocompatibility, low damage on nature corneal stroma, maintaining of structure characteristics of the nature corneal, and maintaining of effective components of the nature corneal so as to provide physical and chemical properties similar to the nature corneal; and after the prepared decellularization cornea is transplanted, animal experiment results show that characteristics of transparent transplanted cornea, no scar formation, no melting generation and no neovascularization are provided, the transplanted cornea and the recipient bed are completely integrated after transplanting a month, the transplanted cornea is subjected to complete epithelization after three months, corneal stroma cells migrate toward the decellularization cornea graft so as to prove that the tissue just takes place slow reconstruction, and the transplanted cornea after 6 months does not show significant difference in histology and appearance detection compared with the nature cornea.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
Decellularized heterogeneous corneal stroma carrier and its preparation method and application
The invention discloses a decellularized heterogeneous corneal stromal carrier and its preparation method and application. The carrier is an animal lamellar cornea from which epithelial cells and stromal cells have been removed through hypertonic solution combined with enzyme digestion. The preparation method of the carrier is as follows: firstly, take Fresh animal eyeballs are aseptically operated under an operating microscope, and the lamellar cornea with a thickness of 150 μm to 400 μm is drilled with a graduated trephine drill with a diameter of 5 mm to 12 mm, and then removed under the combined action of hypertonic solution and trypsin / pancreatin substitute The cells are finally dehydrated and dried to obtain the decellularized heterogeneous corneal stroma carrier, which is stored for future use. The decellularized heterogeneous corneal stroma carrier can be used as a corneal transplant donor to directly perform therapeutic corneal transplantation, and can also be used as an artificial biological corneal scaffold to construct a full-layer or lamellar artificial biological cornea. The decellularized heterogeneous corneal stroma carrier prepared by the invention has the following characteristics: the collagen is neatly arranged, similar to normal corneal tissue, and has good transparency after rehydration.
Owner:陕西省眼科研究所
Method for preparing bovine cornea stroma from fresh bovine cornea and application method
The invention provides a method for preparing a bovine cornea stroma from a fresh bovine cornea. The method comprises the steps that a cornea piece is cut from a fresh bovine eyeball and then steeped in a hypotonic solution; the bovine cornea stroma containing a lamina elastica anterior and part of a stroma layer is obtained from the cornea piece, and is frozen and thawed repeatedly to break cells; the bovine cornea stroma is bleached with a buffer solution, steeped in a cell removing reagent and then washed with ultrapure water in sequence; the bovine cornea stroma is put into another cell removing reagent for steeping, and then washed with ultrapure water again; the bovine cornea stroma is put into a dehydrating agent to be steeped and then irradiated with cobalt-60, and then the prepared bovine cornea stroma is dried and stored. The physical method and low-toxicity regents are adopted in the method, and time is very short, so that the obtained bovine cornea stroma is completely preserved, has the advantages of being good in transparence and compact in structure, and is close to the natural feature of the human cornea.
Owner:北京智慧细胞生物科技有限公司
Aseptic processing preparation method for allogeneic corneal grafts
The invention discloses an aseptic processing preparation method for allogeneic corneal grafts. By virtue of whole treatment on eyeballs, the method comprises the following steps: two-step virus inactivation, namely ultraviolet irradiation and sodium hypochlorite solution soaking, epithelial layer removal, two-step decellularization, namely serum soaking and an osmotic pressure method, cutting and dewatering, and glycerine solution storage. According to the preparation method disclosed by the invention, drying and terminal sterilizing steps are not needed, so that the preparation method is simple in process, short in production cycle and low in production cost; through soft decellularization treatment, the structure and components of the natural corneal stroma are kept to the maximal extent; no significant difference is formed among the transparency, the mechanical strength and the natural corneal stroma; the degradation speed is matched with the regeneration speed of newborn cornea tissues; antigen removal and virus inactivation are thorough; the biocompatibility and the biosecurity are high; the structure and performance of glycerine storage can be kept stable for a long period of time; clinical application is convenient; and corneal transplantation can be carried out instead of the allogeneic cornea.
Owner:SHAANXI BOYU REGENERATIVE MEDICINE CO LTD
Method for decellularization of human corneal stroma
InactiveCN104338181AProtect biological propertiesNo toxicityProsthesisDonor shortageBiological property
The invention aims to solve the problem of donor shortage of corneal stroma and overcome defects of a traditional decellularization method, provides a method for decellularization of human corneal stroma, and belongs to the technical field of cornea in tissue engineering. The method comprises the following steps: collecting corneal stroma containing tissue cells to be put in a cleaning solution, and washing in the cleaning solution for 2-3 times and 2-5 minutes each time; sealing the corneal stroma in nitrogen, and storing for 5-7 days; taking out the corneal stroma from nitrogen, washing by the cleaning solution for 2-3 times and 2-5 minutes each time, shaking at constant temperature in the cleaning solution, changing the cleaning solution every 1-2 hours to obtain decellularized corneal stroma. The method can be used for completely decellularization, collagen of corneal stroma cannot be hurt, preparations applied in the whole process are non-toxic, the biological characteristics of the corneal stroma can be furthest protected, and the stroma cells can be effectively removed.
Owner:沈阳市第四人民医院
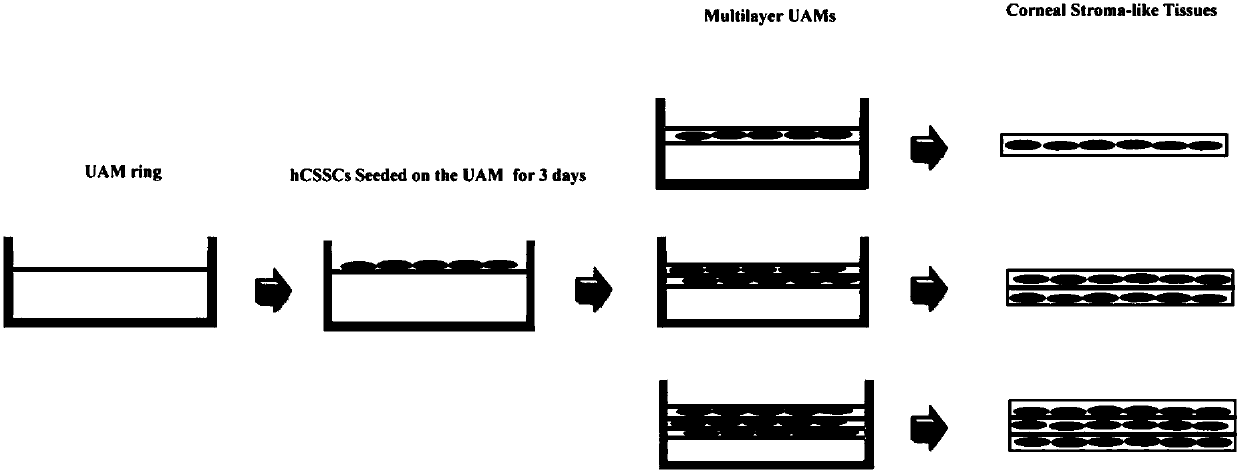
Tissue engineered cornea
InactiveCN107929811AMaintain propertiesAnti-fibrosis propertiesTissue regenerationProsthesisCell-Extracellular MatrixECM Protein
The invention discloses a tissue engineered cornea. The tissue engineered cornea comprises at least three amnion tissue layers, wherein corneal stromal cells are grown between two adjacent amnion tissue layers, extracellular matrixes which are secreted by the corneal stromal cells and contain keratocan, lumican, I type collagen and V type collagen are also contained, and the extracellular matrixesare regularly arranged, so that a tissue structure similar to corneal stroma is formed. Corneal epithelial cells and corneal endothelial cells are respectively inoculated on the upper surface and thelower surface of the structure, so that a total cornea is formed. Surface collagenous fibers of the amnion tissue layers are regularly arranged, are parallel to each other and are transversally intersected and closely arranged to form a fiber sheet, so that the corneal stromal cells and the extracellular matrixes secreted by the corneal stromal cells are guided to be regularly arranged; and at least three amnion tissue layers have the characteristic of inhibiting fibrosis, so that the characteristics of the corneal stromal cells are maintained, and differentiation of the corneal stromal cellsto fibroblasts is inhibited.
Owner:XIAMEN UNIV
Novel biological artificial cornea capable of realizing cellularization through in-vivo induction as well as realizing quick transparency
InactiveCN105688282ACurvature maintenanceQuick fitEye implantsTissue regenerationDepyrogenationBiocompatibility
The invention relates to a novel biological artificial cornea capable of realizing cellularization through in-vivo induction as well as realizing quick transparency. The novel biological artificial cornea is prepared with a method in the steps as follows: 1, obtaining of a cornea raw material; 2, preparation of a cornea scaffold; 3, deep modification of the cornea scaffold; 4, deep inactivation of the cornea for removal of pyrogen; 5, irradiation sterilization of the cornea; 6, cultivation and implantation of corneal endothelial cells. Deep and light lamellar biological artificial corneas with different thicknesses and a full-thickness biological artificial cornea can be established. After being implanted, the novel biological artificial cornea can be quickly healed, becomes transparent quickly and keeps good refraction, the eyesight can be recovered and the eyeball is beautified. The obtained biological artificial cornea has good mechanical performance and biocompatibility, a full-thickness cornea graft is formed after endothelial cells are implanted on a full-thickness cornea stroma scaffold and can be rebuilt in vivo, perform in-vivo induction to promote growth of corneal limbal stem cells and growth of corneal epithelial cells and become transparent quickly on the basis, the treatment efficiency and effects are improved, and an application of lamellar keratoplasty and an application of penetrating keratoplasty are both considered.
Owner:广州宏畅生物科技有限公司
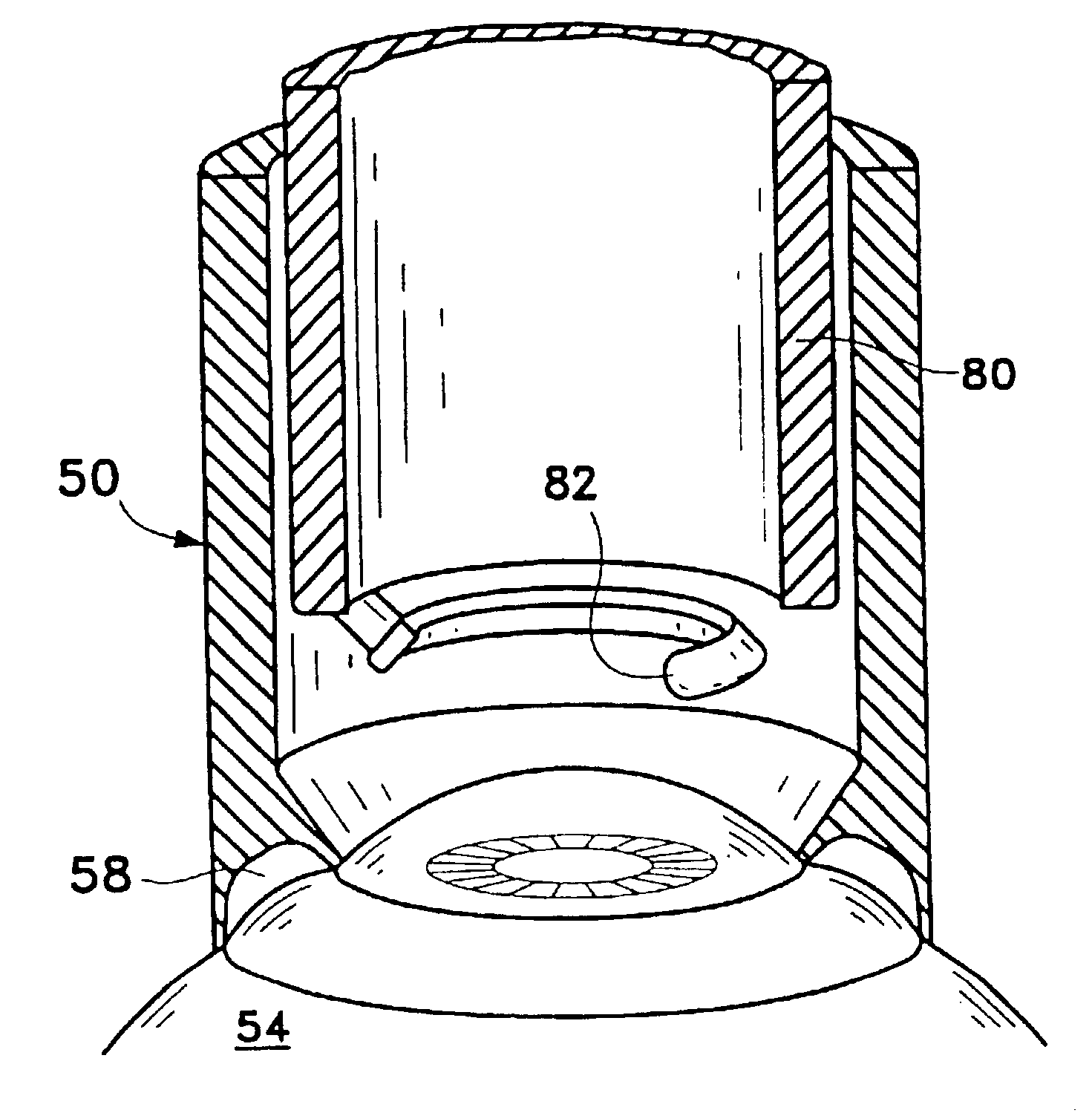
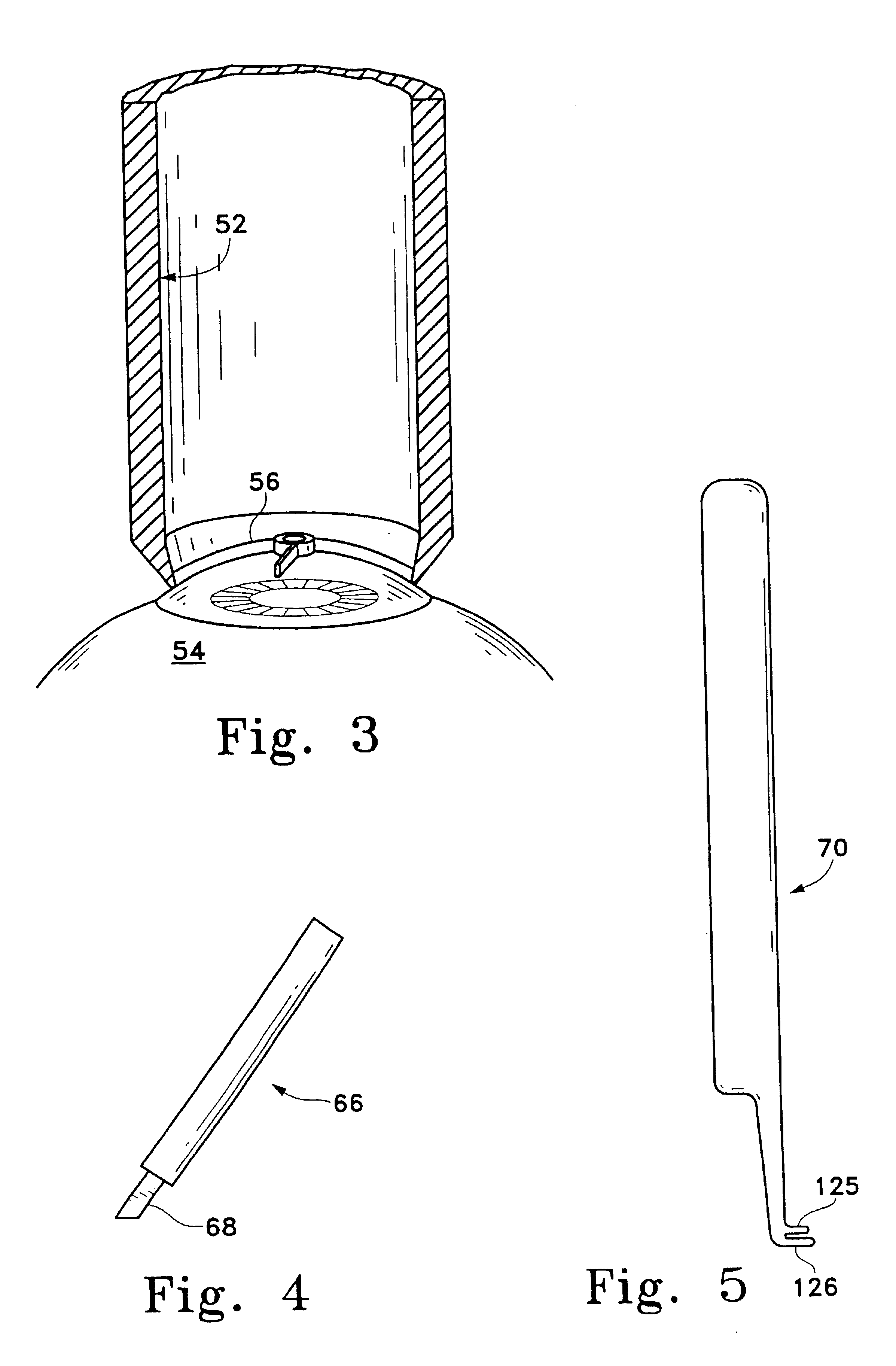
Full embedding bed deep lamellar cornea grafting composite instrument
A combined apparatus for the deep corneal transplantation is composed of two deep corneal matrix hooks consisting of handle and the extended rod with hook head, and a viscoelastic agent injector consisting of needle seat and tube. Its advantages are short operation time and high safety and effect.
Owner:THE AFFILIATED SIR RUN RUN SHAW HOSPITAL OF SCHOOL OF MEDICINE ZHEJIANG UNIV
Soft and transparent silk fibroin membrane and preparation method thereof
ActiveCN106700566AGuaranteed biocompatibilityPersistent transparencyBiocompatibility TestingOxygen permeability
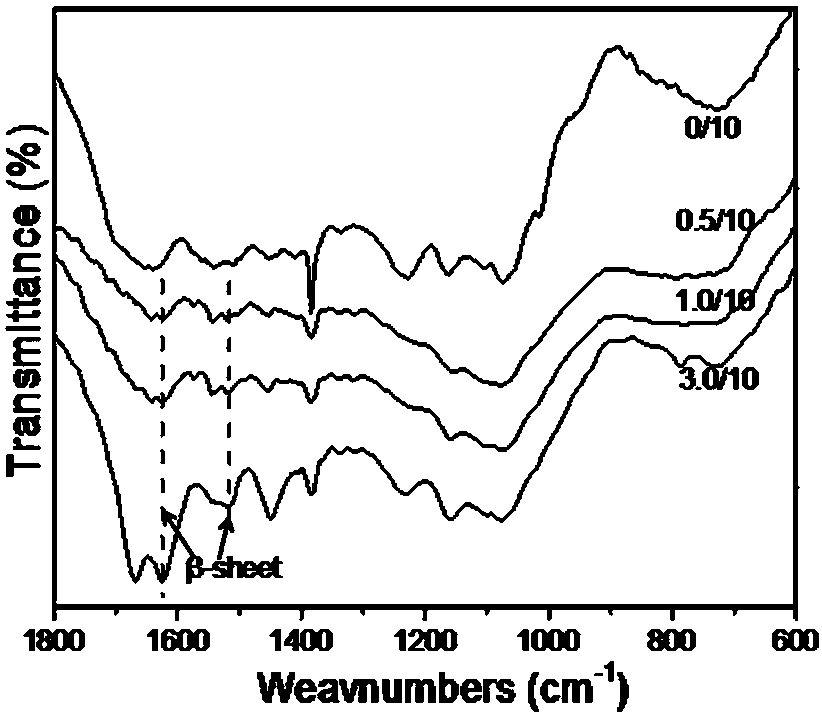
The invention discloses a soft and transparent silk fibroin membrane and a preparation method thereof. The preparation method comprises the following steps: taking silkworm fibroin as a base material, blending non-bio-toxic small molecule urea and a silk fibroin aqueous solution, pouring at a room temperature, drying under a certain humidity and balancing into a membrane to prepare the silk fibroin membrane that is soft, is insoluble in water and is kept transparent for a long time in dry and wet states. The light transmittance of the silk fibroin membrane with a thickness of about 100 [mu]m within a visible light range of 400-760 nm in the dry and wet states is 96 percent or above; when the dry-state elongation at break is 75 percent or above, the breaking strength is 25 MPa or above; when the wet-state elongation at break is 120 percent or above, the breaking strength reaches 10 MPa or above. The dissolve-loss ratio in deionized water is 0.5 percent or below. Due to excellent biocompatibility of natural silk fibroin and better oxygen permeability and hydrophilicity of a fibroin membrane, the silk fibroin membrane is particularly suitable for the repair of corneal stroma and the application to contact lens, eye pupil materials and the like.
Owner:NANTONG TEXTILE & SILK IND TECH RES INST
Method of Tissue-Selective Targeted Gene Transfer
The present invention relates to methods of delivering a gene, such as a therapeutic gene, to a desired area of stroma of a cornea that involves removing the corneal epithelium and dehydrating the cornea. Certain aspects of the present invention relate to methods of treating corneal scarring by delivering a TGFβ-antagonizing gene packaged in a viral vector.
Owner:UNIVERSITY OF MISSOURI
Acellular cornea stroma lens and preparation method thereof
InactiveCN107233143AImprove securityImprove performanceIntraocular lensPermanent implantImmunogenicity
The invention provides an acellular cornea stroma lens and a preparation method thereof, and relates to the technical field of tissue engineering. By means of the preparation method of the acellular cornea stroma lens, cellular constituents in stromata can be effectively removed, the immunogenicity of the cornea stroma lens is reduced, the mechanical strength of the cornea stroma lens is improved, and moreover, the original morphology and the transparency of the cornea stromata can be effectively kept. The prepared acellular cornea stroma lens has the advantages of being higher in safety, better in transparency and more stable in performance; the lens can be used as a scaffold for the proliferation and growth of cornea stroma cells, and finally, the lens and self-body corneas are fused together and serves as a permanent implant lens; besides, the phenomena of immunological rejection and the like can be avoided.
Owner:GUANGZHOU SUN SHING BIOTECH CO LTD
Cosmetic corneal inlay and implantation method thereof
ActiveUS20170319330A1Normalize structural anomalySolve the real problemEye implantsMetaboliteCorneal inlay
Disclosed is an intrastromal insert, already preformed, of solid, transparent, impermeable, biocompatible, physiologically inert and chemically resistant material, with notches allowing the passage of metabolites, substances, cells or portions thereof, and drugs, configured to receive printing, which is adapted in the space of the corneal stroma to change the eye color and / or to solve the problem of insufficient pigmentation of the iris. The method and the related component to change, permanently and reversibly, the eye color for aesthetic purposes are described as well.
Owner:OPHTA INNOVATIONS
Cornea matrix, preparation method and application
ActiveCN107050515AAchieve swellingIncrease spacingTissue regenerationProsthesisInfectious KeratitisCell-Extracellular Matrix
The invention provides a cornea matrix, a preparation method and an application. An animal cornea is used as a raw material; a cornea epithelial layer and cell components and DNA components in the cornea matrix are removed; a lamina elastica anterior and a local thick cornea matrix are reserved; and complete extracellular matrixes are reserved. The cornea matrix is suitable for covering of a pathological cornea, especially is suitable for patients suffering from unperforated corneal ulcer untreatable with drugs and infectious keratitis requiring interlayer transplantation therapy, and is suitable for temporary coating of corneal perforation.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
In-vitro construction method of tissue engineering posterior lamellar cornea
PendingCN111110921AGood growthLow antigenicityNervous system cellsCell culture supports/coatingCorneal endothelial cellTissue architecture
The invention relates to an in-vitro construction method of a tissue engineering posterior lamellar cornea. The tissue engineering posterior lamellar cornea includes human corneal stromal cells and human corneal endothelial cells which are adopted as corneal seed cells and an acellular porcine corneal matrix which has a posterior elastic layer and is adopted as a tissue engineering corneal carrierscaffold, wherein the in-vitro construction method of the tissue engineering posterior lamellar cornea is characterized by comprising the steps: inoculating the corneal stromal seed cells to the scaffold firstly so as to construct the stromal layer part of the tissue engineering posterior lamellar cornea, and then inoculating the corneal endothelial seed cells to the posterior elastic layer so asto construct the tissue engineering posterior lamellar cornea which has a tissue structure and functions similar to the tissure structure and functions of a normal posterior lamellar cornea. The international problem is solved that human corneal endothelial cells cannot be proliferated in vitro, and a high-density and tight-connection single-layer endothelial structure with 3400-3600 cells / mm<2>is formed by the corneal endothelial cells; and an injection inoculation method is adopted for inoculation of the corneal stromal cells, not only can the in-vitro culture time be shortened greatly, but also the structure integrity of the carrier scaffold can be maintained; and therefore, needs of high quality and low cost can be met, and mass production and promotion and application are achieved.
Owner:OCEAN UNIV OF CHINA
Corneal endothelial membrane based on human corneal stroma as carrier
PendingCN112156228AMaximize valueOxygen permeableTissue regenerationProsthesisCorneal endothelial cellCorneal endothelium
The invention relates to a corneal endothelial membrane based on a human corneal stroma as a carrier. The average thickness of the human corneal stroma as the carrier is 20-100 [mu]m, preferably 20-70[mu]m; the carrier is provided with a modification layer for blocking water and promoting cell adhesion, and the modification layer is inoculated with corneal endothelial cells. By adopting the humancorneal stroma with smaller average thickness, one donor material can be cut into a plurality of slices, so that the maximum value of the donor material is exerted, and a good stent material can be provided for the construction of the endothelial membrane.
Owner:PEKING UNIV THIRD HOSPITAL
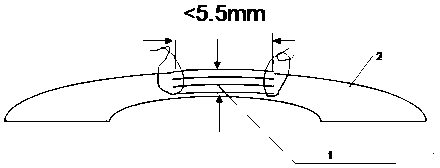
Corneal lamellar piece and production method thereof
InactiveCN103892938AReduce waiting timeLower surgery costsEye implantsFemto second laserRefractive surgery
The invention relates to the fields of biology and medicine, particularly relates to the field of ophthalmic medicine, in particular to a corneal lamellar piece and a production method thereof, and aims to solve the problem existing in current treatment repairing. The corneal lamellar piece is formed by stacking stromal corneal lens units, fibrin adhesive layers are arranged among the stromal corneal lens units, the maximum thickness of the corneal lamellar piece ranges from 350 to 450micrometer, and the diameter of the corneal lamellar piece ranges from 6 to 6.5mm. The stromal corneal lens units are made from operation garbages of femtosecond laser corneal refractive operations, so that the operation garbages are converted into repairing materials urgently needed in eyeball treatment repairing, the problem that material waiting period in eyeball treatment repairing is long is solved, operation cost is reduced greatly, and the corneal lamellar piece is significant in the ophthalmic medical field.
Owner:薛春燕
Highly-transparent acellular corneal stroma and preparation method thereof
ActiveCN111686301AAvoid damageMaintain normal physiologyTissue regenerationProsthesisThree dimensional microstructureBowman's layer
The invention discloses a preparation method of a highly-transparent acellular corneal stroma. The preparation method comprises the following steps: 1, cleaning and virus inactivation treatment are carried out on a picked animal eyeball; 2, a cornea is shorn, and then the shorn cornea is cleaned; 3, the cornea is put into a hypotonic solution, subjected to ultrasonic treatment and then transferredinto a shaking table for shaking treatment; 4, the cornea is repeatedly treated 3-4 times; 5, hypertonic and hypotonic solution treatment is carried out, after every 1-2 times of hypertonic and hypotonic solution treatment, the cornea is put into a transparent solution to be treated for 0.5-3 h, and after the last time of hypertonic and hypotonic solution treatment, the cornea is dehydrated; 6, the dehydrated cornea is cut; and 7, the cut cornea is sterilized and then preserved. According to the method, the corneal stroma can be effectively prevented from absorbing water and swelling, collagen bundles and a natural three-dimensional microstructure are prevented from being damaged, therefore it is guaranteed that collagen structures of a Bowman's membrane and a stroma layer are arranged inorder, and the prepared acellular corneal stroma has the optical performance and strength similar to those of a fresh cornea.
Owner:SHAANXI BOYU REGENERATIVE MEDICINE CO LTD
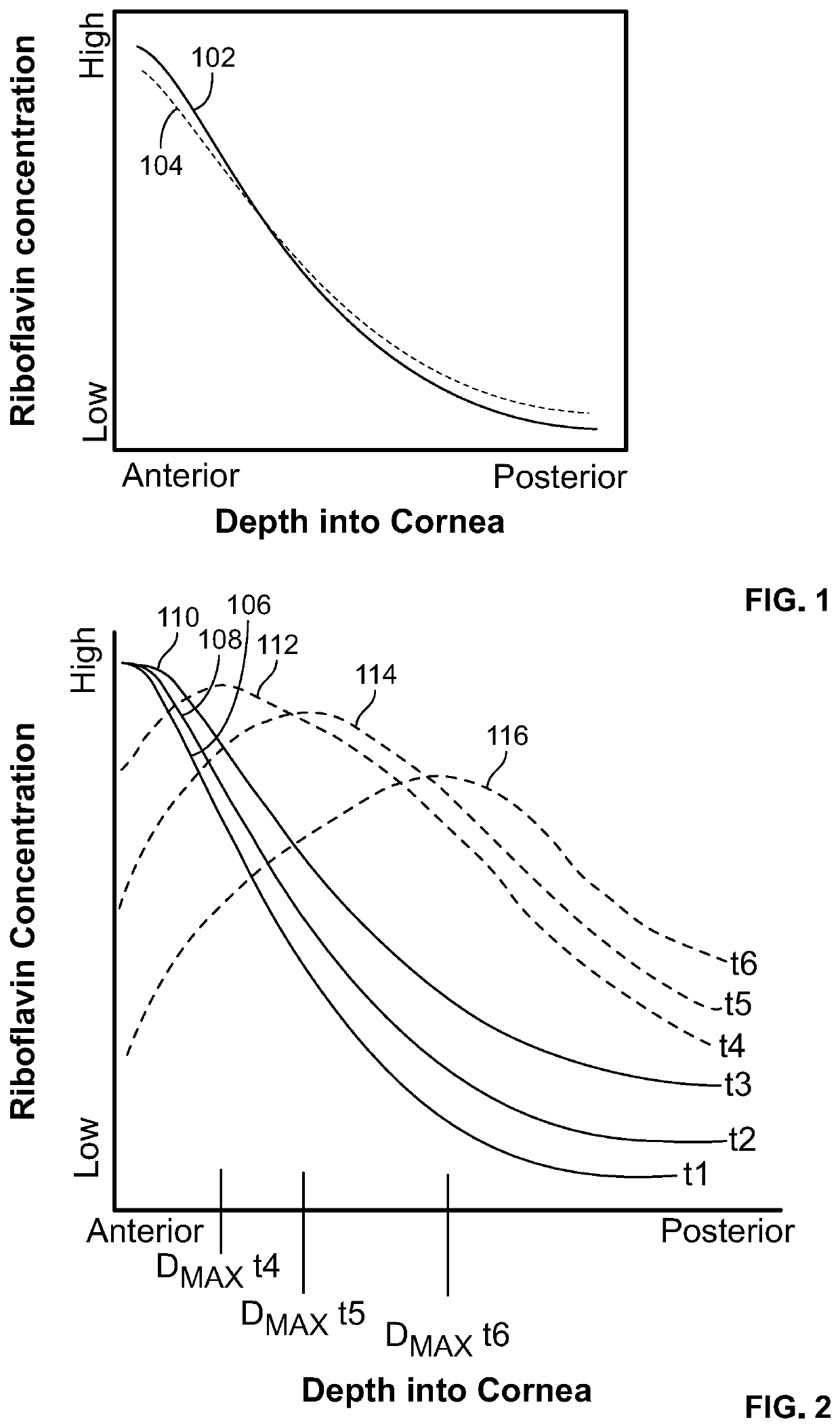
Corneal Crosslinking With Catalyst Distribution Control
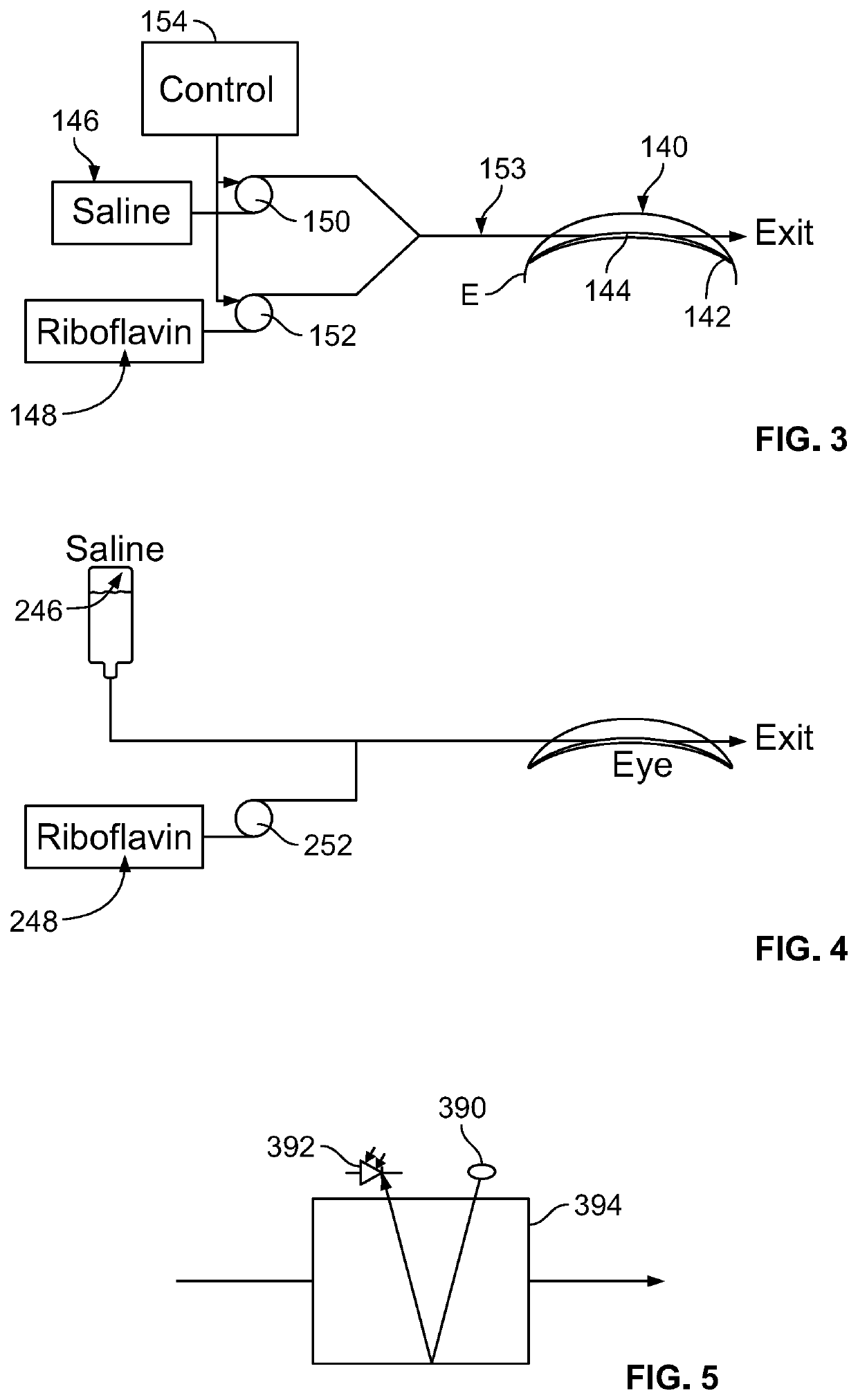
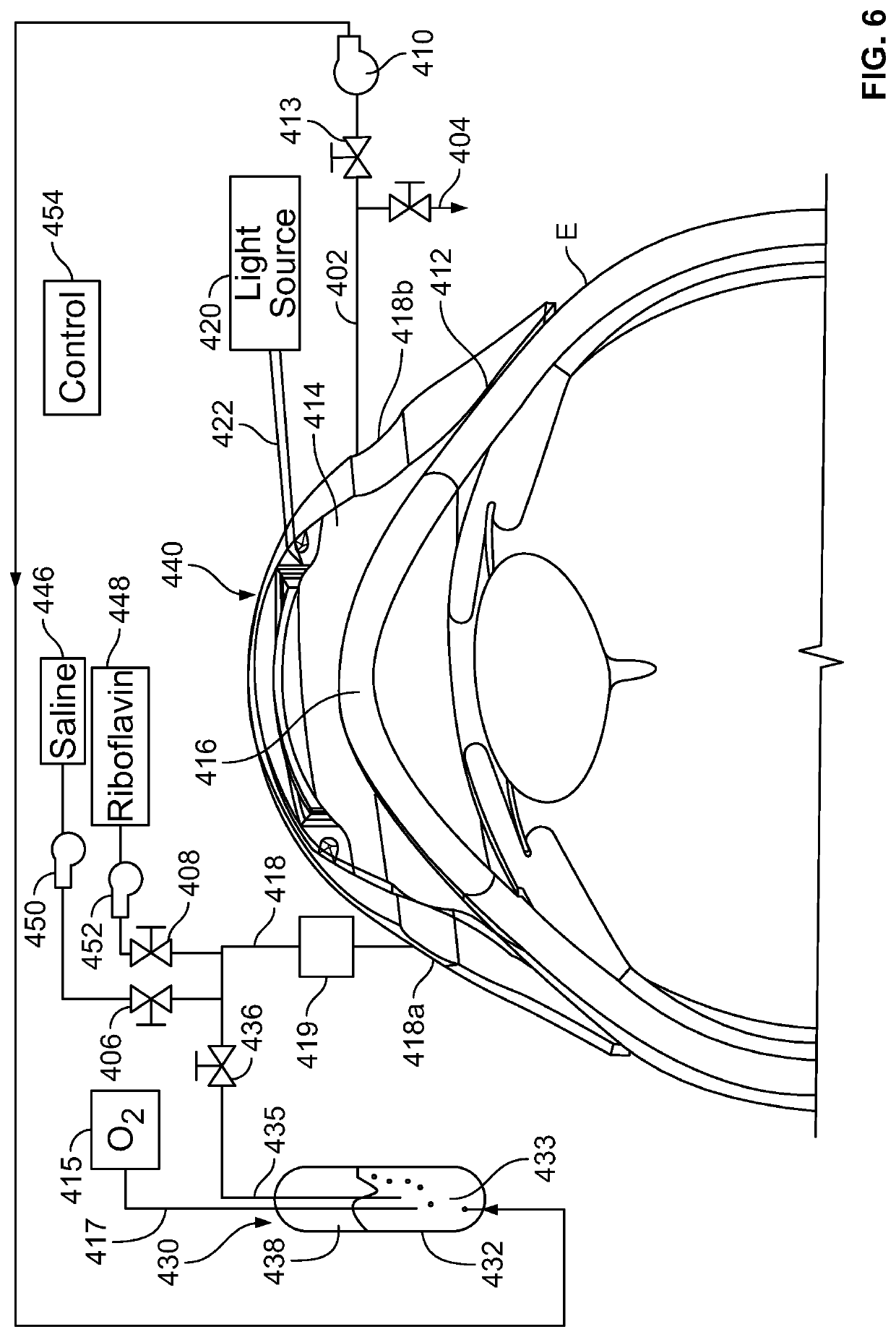
In corneal crosslinking, the anterior surface of the cornea of the eye is maintained in contact with a first liquid having a first concentration of a crosslinking catalyst such as riboflavin, so that the catalyst enters the cornea and forms a first concentration profile (t1) in the corneal stroma. The anterior surface of the cornea is then maintained in contact with one or more additional liquids having concentration of the catalyst lower than the first concentration so that the catalyst forms a second concentration profile (t4, t5, t6) in the stroma. In the second concentration profile, the maximum concentration of the catalyst desirably is posterior to the anterior surface of the cornea. The cornea is irradiated and crosslinked. The second concentration profile facilitates crosslinking deep within the stroma.
Owner:TECLENS
Corneal stromal keratocyte culture
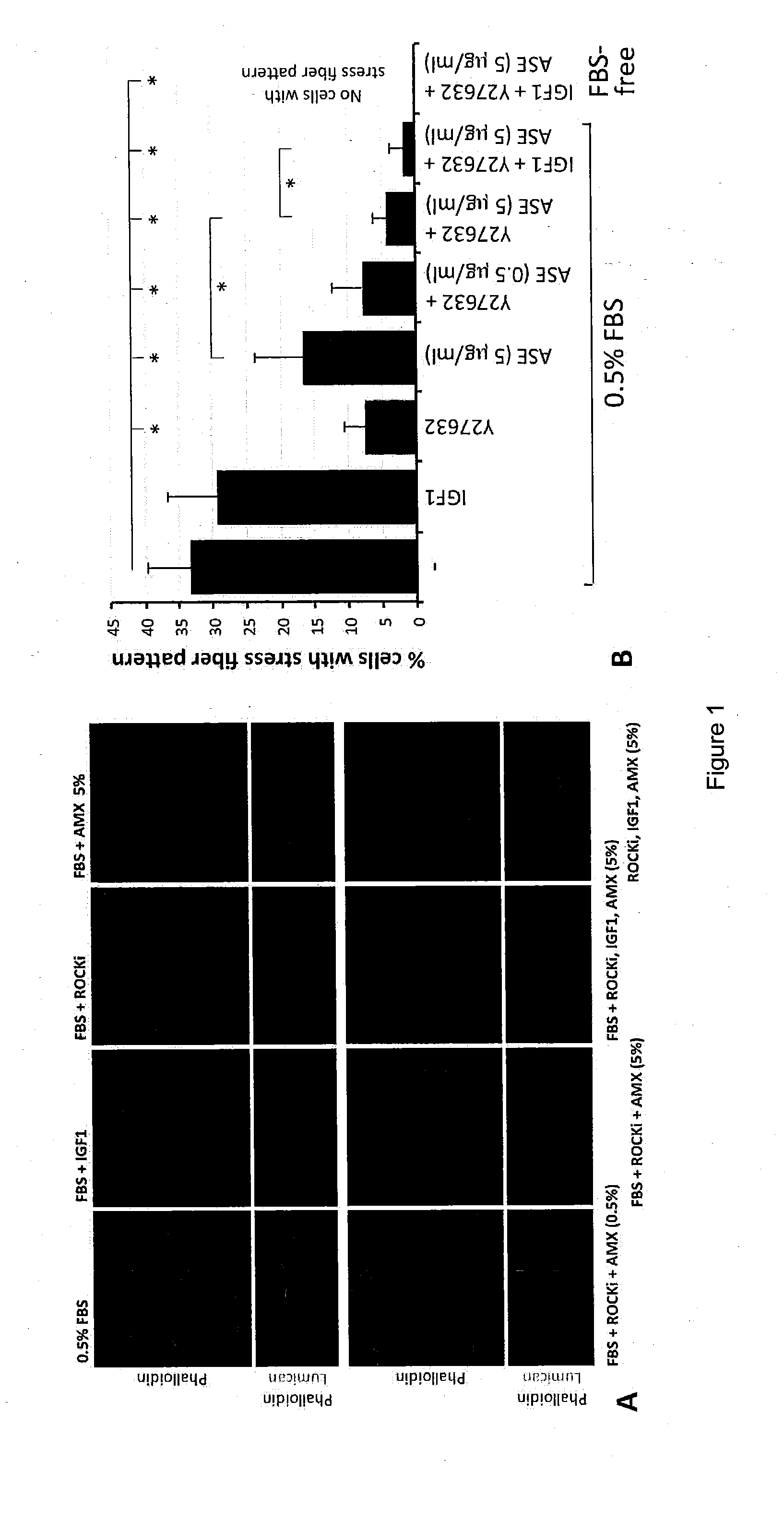
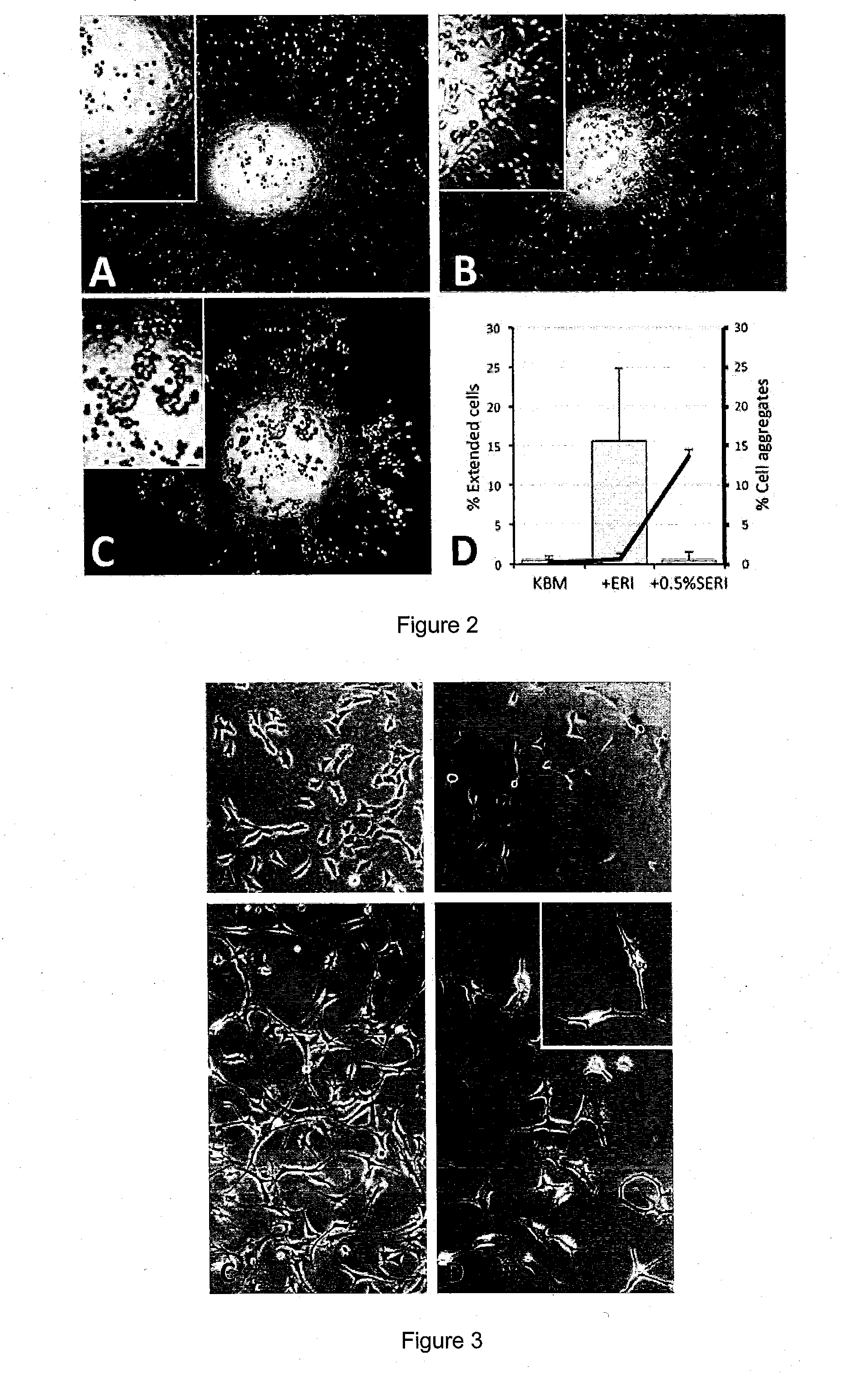
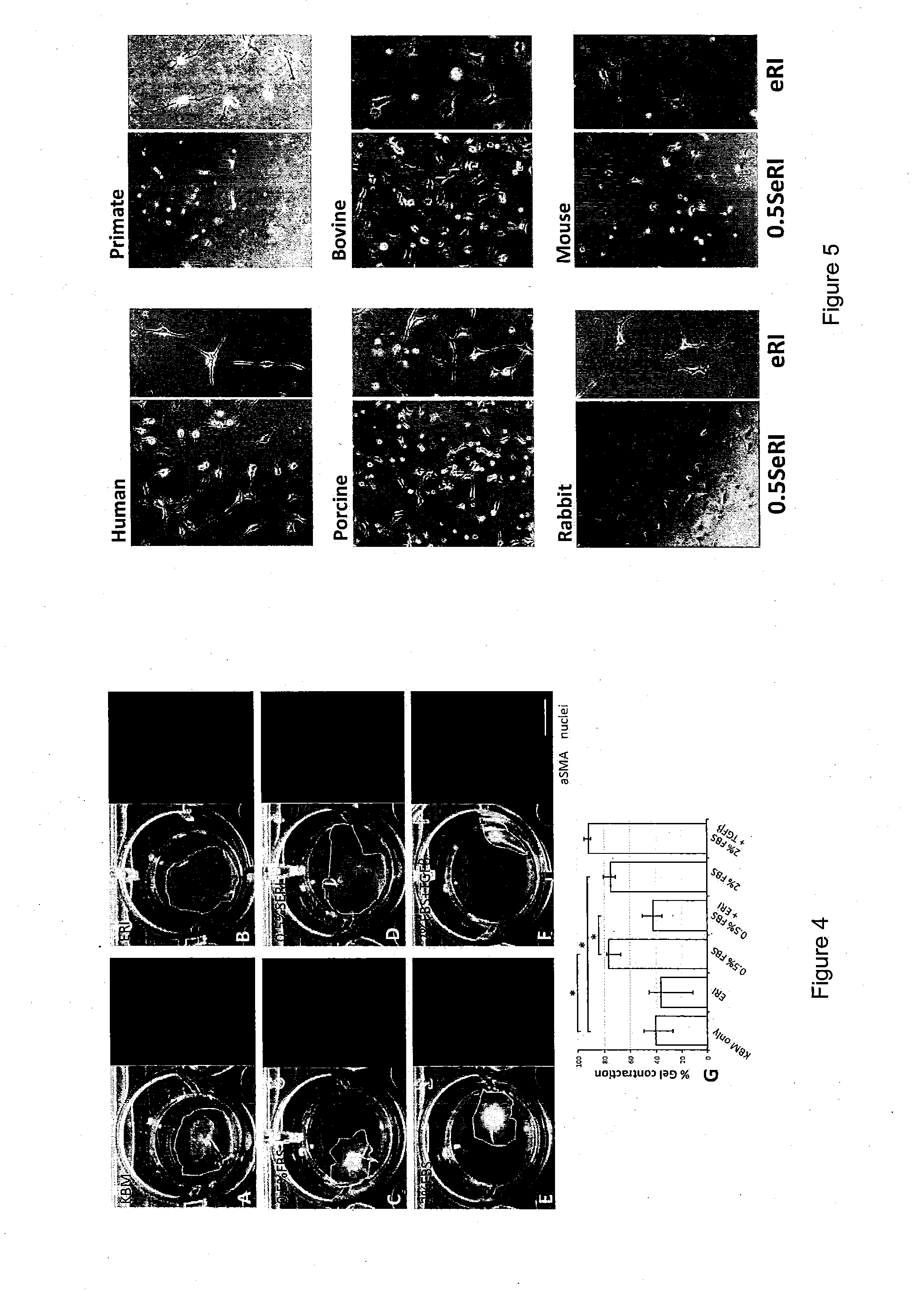
The present invention relates to corneal stromal keratocytes (CSKs) culture. In particular the present invention relates to a method for culturing corneal stromal keratocytes ex vivo. The method utilises a dual culture medium protocol. In the presence of serum (in particular low serum concentration) in a first culture medium A, the “activated keratocytes” are expandable while specific CSK gene expression is maintained in serum-free ERI condition in a second culture medium B. The invention also relates to culture medium A and B which is supplemented with liquid amnion extract or other additional supplements. This protocol also applied to CSK from other species. Additionally, the present invention provides method for CSK cultivation to provide sufficient quantity of genuine CSKs for corneal tissue engineering without a risk of fibroblastic changes.
Owner:SINGAPORE HEALTH SERVICES PTE
Artificial cornea
The invention relates to an artificial cornea, and in particular relates to the artificial cornea that does not easily fall out, prevents the leakage of aqueous humor, and is beautiful and natural. The artificial cornea includes a central optical part and an umbrella wing is proposed, the central optical part is a round light-transmitting part, and the side surface is rough; the edge of the central optical part includes at least two fixing feet, and the fixing feet are used to be inserted into a corneal stroma of a graft bed to fix the central optical part and prevent the central optical partfrom falling out; the center of the umbrella-shaped wing is a circular hole and is arranged relative to the circular light-transmitting part, and the diameter of the central circular hole of the umbrella-shaped wing is less than or equal to the diameter of the circular light-transmitting part; the outer side of the central circular hole of the umbrella-shaped wing is an annular leak-proof zone; and the outer side of the umbrella-shaped wing annular leak-proof zone includes at least two side wings, and each side wing includes a biointegration zone for biointegration with a conjunctiva.
Owner:姚晓明
Stabilization of collagen scaffolds
Shape-stabilized collagen scaffolds and methods of obtaining such scaffolds are disclosed. Stroma can be harvested, for example, from human or porcine corneal stroma and shaped during excision or in a separate step after excision. Following shaping (and preferably decellularization), the excised stroma portion is subject to pressure, force or vacuum to reduce fluid content and then irradiated or otherwise treated to induce crosslinking of collagen chains or fibrils. In one embodiment, the scaffold can be compacted by removing some or all of the water from the scaffold, and rehydrating the scaffold in a controlled manner (e.g., in a mold or other confining space) such that the scaffold takes a desired compacted shape; and then crosslinking at least a portion of the scaffold to mechanically strengthen it and inhibit subsequent swelling. Various sources of energy can be employed to induce crosslinking of collagen including, for example, ultraviolet (UV) radiation. The scaffolds can also be selectively densified or patterned. The invention is particularly useful in forming stable lenticules of enhanced stiffness and sufficient optical clarity for intracorneal implantation in additive ocular surgery.
Owner:GEBAUER KLOPOTEK PATENT VERWALTUNG UG
Corneal stroma injection needle
PendingCN114129342AGuaranteed oblique penetrationEasy to observeEye surgeryNeedle insertionStroma of cornea
The invention provides a corneal stroma injection needle which comprises a needle body, the needle body comprises a hollow needle tube body and a needle tip located at the front end, a movable supporting piece and a limiting piece are arranged on the needle tube body, and the limiting piece limits the distance between the supporting piece and the needle tip to be constant. Due to the fact that the tube diameter of the needle tube body at the position where the supporting piece is additionally arranged is increased, and the bottom face of the supporting piece is arc-shaped, the contact face of the supporting piece and the cornea of the eyeball is close to point contact when the supporting piece abuts against the cornea of the eyeball, an operator can conveniently observe and adjust the included angle between the needle tube body and the supporting piece, and it is guaranteed that the needle tube body can obliquely penetrate into the cornea. And the limiting piece is used for enabling the length from the supporting piece to the needle point to be a preset value before puncture, so that an operator can conveniently determine the initial position before needle insertion, and the needle insertion depth can be conveniently designed according to the inclination angle of the needle tube body.
Owner:何宏 +1
Shaped corneal segments: corneal allogenic intra-stromal devices (ring segments and rings, modified discs, modifications) for inducing shape change, regularization and stabilization of cornea in corneal ectasia and other corneal conditioins and for correction of refractive errors
A device for implantation into the cornea intra-stromally comprising allogenic comeal or scleral material or other bioengineered material including, but not limited to, processed collagen tissues, comprises a segment that is inserted into a comeal channel whereby the segment regularizes the conical cornea, gives an improved surface, improves biomechanical strength distribution and stability improves optical functionality, and improves / corrects the refractive error or gives other desired shape change effects.
Owner:JACOB SOOSAN
Artificial cornea frame, artificial cornea stroma and preparation method thereof
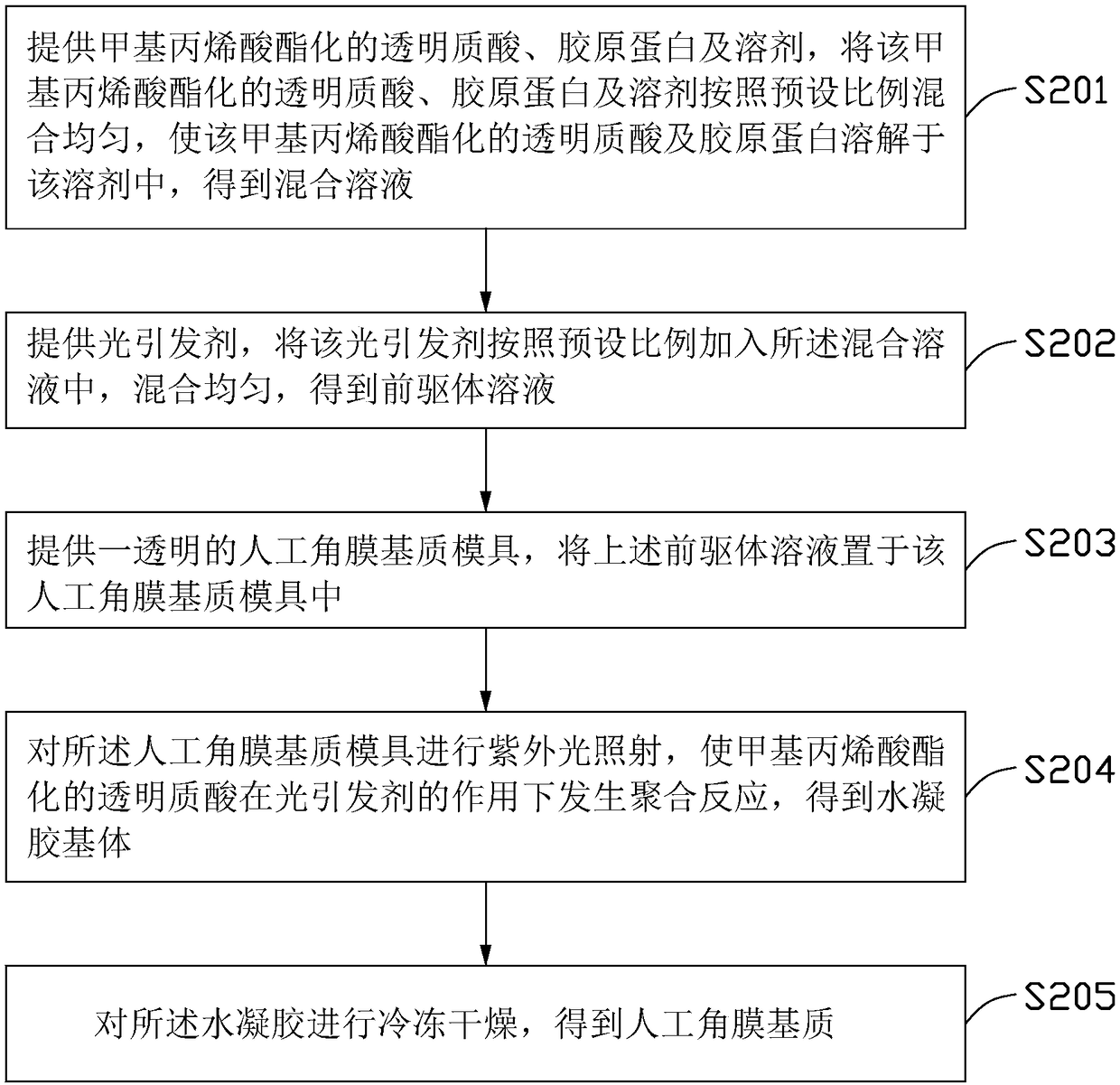
The invention relates to an artificial cornea stroma. The artificial cornea stroma is made of an artificial cornea stroma material. The artificial cornea stroma material comprises methacrylated hyaluronic acid, collagen and a photoinitiator. The artificial cornea stroma has nano patterns on the surface. The invention further relates to a method for preparing the artificial cornea stroma and an artificial cornea frame with the artificial cornea stroma.
Owner:HONG FU JIN PRECISION IND (SHENZHEN) CO LTD +1
Preparation method of ultrathin APCS implant
ActiveCN111035807AReduce manufacturing costLow costTissue regenerationProsthesisOphthalmologyReoperative surgery
The invention discloses a preparation method of an ultrathin APCS implant and relates to the field of corneal endothelial sheets. The preparation method comprises the following steps: cleaning and dehydrating an acellular porcine corneal stroma, pre-cooling a freezing slicer to be lower than 20 DEG C, smearing an OCT embedding agent on a sample support, carrying out flattening, placing the dehydrated stroma on the OCT embedding agent, carrying out freezing, setting the slice thickness of the freezing slicer to be 20-60 microns, and carrying out cutting to obtain the ultrathin APCS implant slice . According to the method, the ultrathin APCS implant with the thickness of 20-60 microns is obtained, the integration of the implant and the eyes of a patient is effectively improved, the success rate of an operation is increased, the cost of the freezing slicer is low, the operation method is simple, the preparation cost of the implant can be effectively reduced, and the medical cost of the patient is reduced.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com