Aromatic terminal hydroxyl chain extender containing acylhydrazone bond, self-repairing polyurethane and preparation method thereof
A hydroxyl-terminated chain extender and aromatic technology, which are used in the field of self-healing polyurethane and its preparation, self-repairing polyurethane, and aromatic hydroxyl-terminated chain extender, can solve the problem that repair units are not easy to uniformly disperse, unfavorable for long-term use of materials, and loss of self-repair. Repair function and other problems, to achieve the effect of good physical and mechanical properties, high self-repairing efficiency, and good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0030] Example 1.1: Preparation of an aromatic hydroxyl-terminated chain extender containing an acylhydrazone bond
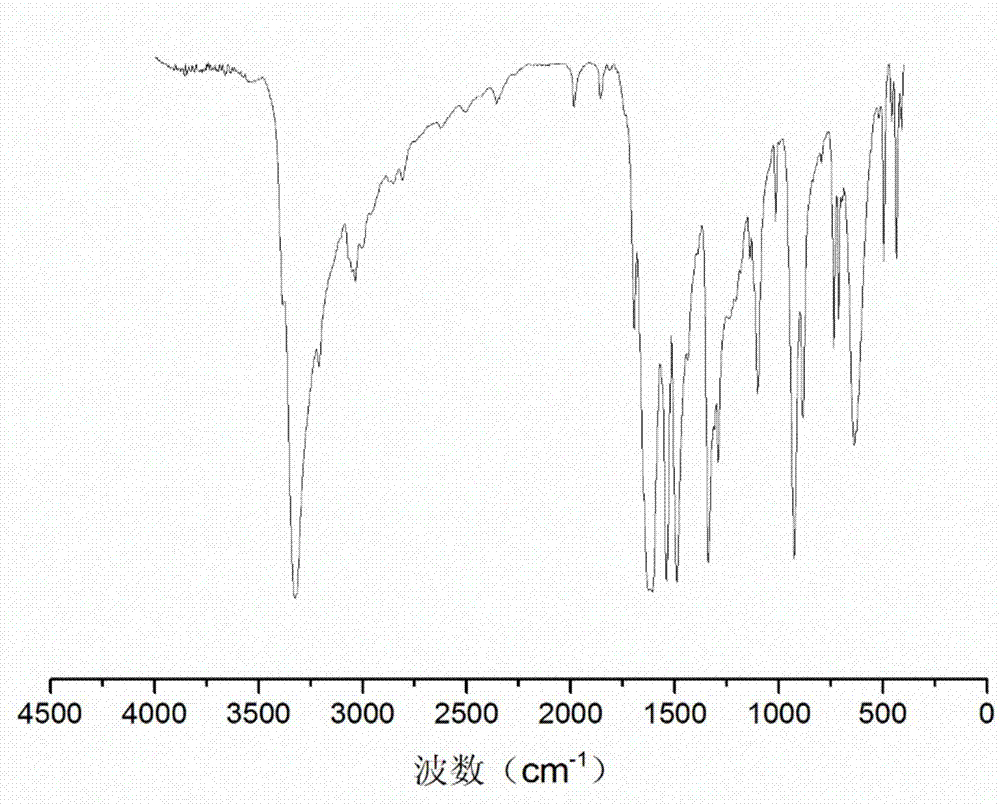
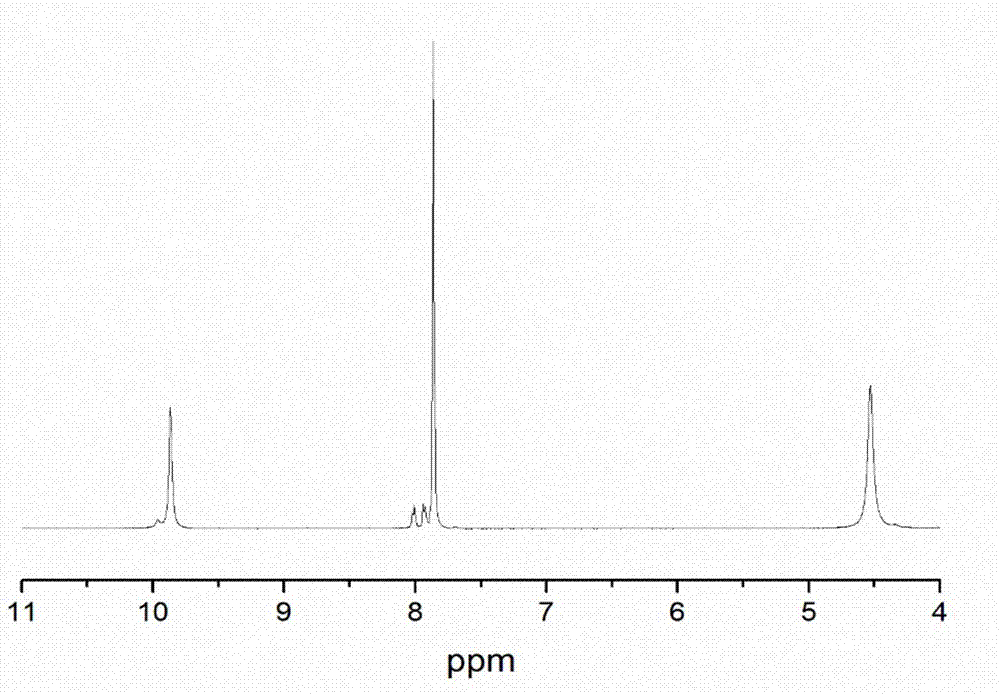
[0031] Take 23.23g of 4-hydroxy-4-methyl-2-pentanone and dissolve in 50.00g of deionized water, and dissolve 19.42g of terephthalic acid dimethylhydrazide in 70.00g of glacial acetic acid. After mixing the two evenly, react at 70°C for 2h under stirring at 250rpm. Filtrate under reduced pressure, and vacuum-dry the resulting product to obtain an aromatic hydroxyl-terminated chain extender containing an acylhydrazone bond. The molecular structure is as shown in formula (1), named 4-hydroxy-4-methyl-2-pentanone Terephthalic acid diformylhydrazone, the product is a white solid powder. Obtain product 33.20g, productive rate 85%, its infrared spectrogram is as image 3 As shown, its NMR spectrum is shown as Figure 4 shown.
[0032]
Embodiment 12
[0033] Example 1.2: Preparation of an aromatic hydroxyl-terminated chain extender containing an acylhydrazone bond
[0034] Dissolve 13.81g of 2,5-dihydroxybenzaldehyde in 90.00g of isopropanol, and dissolve 16.82g of 3,5-dihydroxyphenylhydrazide in 30.00g of glacial acetic acid. Mix the two evenly and react at 50° C. for 4 hours under stirring at 250 rpm. Filtrate under reduced pressure, and vacuum-dry the obtained product to obtain an aromatic hydroxyl-terminated chain extender containing an acylhydrazone bond. The molecular structural formula is shown in formula (2), and it is named as 2,5-dihydroxybenzaldehyde Dihydroxybenzoylhydrazone, the product is light yellow solid powder. The product was obtained in 23.72 g, yield 82.3%.
[0035]
Embodiment 13
[0036] Example 1.3: Preparation of an aromatic hydroxyl-terminated chain extender containing an acylhydrazone bond
[0037] Dissolve 27.62g of salicylic acid in 70.00g of deionized water, and dissolve 42.00g of 3,5-dihydroxyphenylhydrazide in 50.00g of glacial acetic acid. Mix the two evenly and react at 65° C. for 2.5 hours under stirring at 250 rpm. Filtrate under reduced pressure, and vacuum-dry the obtained product to obtain an aromatic hydroxyl-terminated chain extender containing an acylhydrazone bond. , the product is light yellow solid powder. 44.68 g of the product was obtained with a yield of 77.5%.
[0038]
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation at break | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com