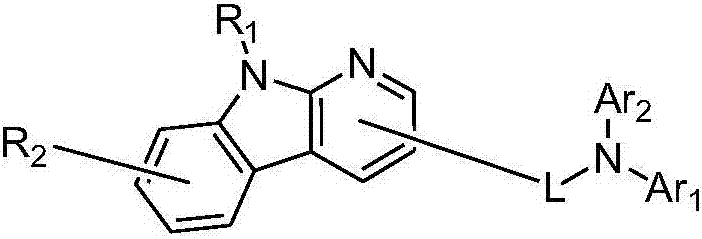
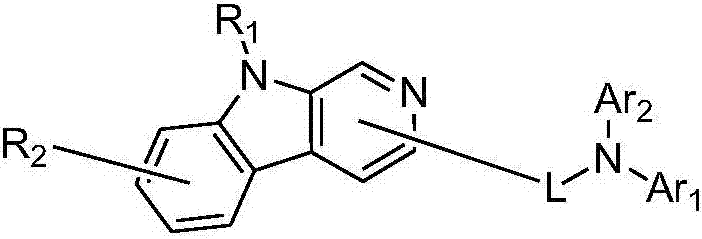
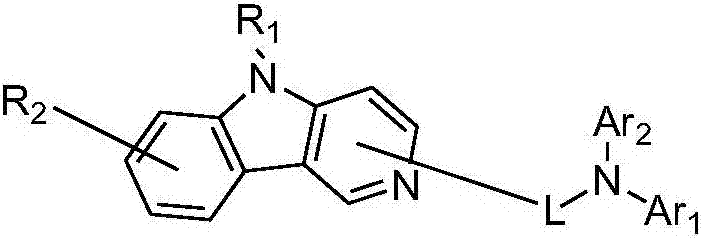
Pyridoindolium compound and organic light-emitting device thereof
A technology of indole compounds and organic compounds, applied in the direction of light-emitting materials, organic chemistry, electric solid-state devices, etc., can solve the problems that cannot be improved, and the luminous efficiency is reduced, so as to achieve good luminous efficiency, improve hole mobility, and high brightness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Embodiment 1, the preparation of compound 2-37
[0093]
[0094] Add B-3 (4.87g, 16.9mmol), A-1 (9.34, 16.9mmol), tetrakistriphenylphosphine palladium (0.7g, 1.08mmol), potassium carbonate (5.3g, 38.3mmol), toluene 500mL in the reaction vessel , 40mL of ethanol and 40mL of distilled water, stirred at 120°C for 3h. After the reaction was completed, stop the reaction with distilled water and extract with ethyl acetate. The organic layer was dried over MgSO4. The solvent was distilled off under reduced pressure and then purified with a silica gel column to obtain compound 2-37 (6.05 g, 50%).
Embodiment 2
[0095] Embodiment two, the preparation of compound 3-37
[0096]
[0097] Add C-3 (4.87g, 16.9mmol), A-1 (9.34, 16.9mmol), tetrakistriphenylphosphine palladium (0.7g, 1.08mmol), potassium carbonate (5.3g, 38.3mmol), toluene 500mL in the reaction vessel , 40mL of ethanol and 40mL of distilled water, stirred at 120°C for 3h. After the reaction was completed, stop the reaction with distilled water and extract with ethyl acetate. The organic layer was dried over MgSO4. The solvent was distilled off under reduced pressure and then purified with a silica gel column to obtain compound 3-37 (5.81 g, 48%).
Embodiment 3
[0098] Embodiment three, the preparation of compound 4-37
[0099]
[0100] Add D-3 (5.12g, 16.9mmol), A-1 (9.34, 16.9mmol), tetrakistriphenylphosphine palladium (0.7g, 1.08mmol), potassium carbonate (5.3g, 38.3mmol), toluene 500mL in the reaction vessel , 40mL of ethanol and 40mL of distilled water, stirred at 120°C for 3h. After the reaction was completed, stop the reaction with distilled water and extract with ethyl acetate. The organic layer was dried over MgSO4. The solvent was distilled off under reduced pressure and then purified with a silica gel column to obtain compound 4-37 (5.55 g, 45%).
[0101] Table 1 shows the FD-MS values of the pyridoindole compounds prepared in the examples of the present invention.
[0102] [Table 1]: FD-MS values of pyridoindole compounds
[0103] compound
FD-MS
2-37
m / z=715.3 (C53H37N3=715.88)
3-37
m / z=715.3 (C53H37N3=715.88)
4-37
m / z=729.31 (C54H39N3=729.91)
[0104] Device Prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com