Hepatitis C virus RNA (ribonucleic acid) extracting kit
A hepatitis C virus and nucleic acid extraction reagent technology, which is applied in the field of biomedical detection, can solve the problems of long window period and long-term existence, and achieve the effect of high safety, efficient extraction and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of Plasma HCV Extraction Reagent
[0038] The following four formulations were prepared for the extraction of plasma HCV.
[0039] Formulation A: kit of the present invention
[0040] Nucleic acid extraction reagent 1: proteinase K 20mg / mL;
[0041] Nucleic acid extraction reagent 2 (lysate): guanidine hydrochloride 573.18g / L, triton (TX-100) 200ml / L, citric acid 1.68g / L, sodium citrate 7.35g / L, pH 4.4±0.1;
[0042] Nucleic acid extraction reagent 3: magnetic beads, 100%;
[0043] Nucleic acid extraction reagent 4 (washing liquid I): Guanidine hydrochloride 382.12g / L, citric acid 1.05g / L, sodium citrate 11.76g / L, isopropanol 400mL / L, pH is 5.4 ± 0.1;
[0044] Nucleic acid extraction reagent 5 (washing solution II): sodium chloride 5.84g / L, Triton (TX-100) 10ml / L;
[0045] Nucleic acid extraction reagent 6: mineral oil 100% (v / v);
[0046] Nucleic acid extraction reagent 7: 1MTris (pH 8.0) 10ml / L, 0.5M EDTA (pH 8.0) 2mL / L.
[0047] During the preparatio...
Embodiment 2
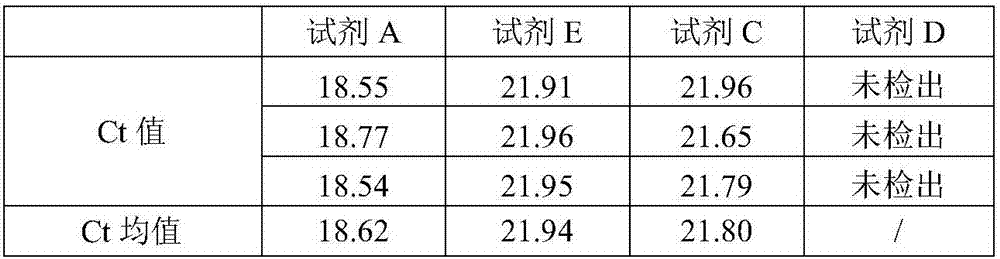
[0063] Comparison of Extraction Efficiency of Different Formulas
[0064] HCV plasma samples with a concentration of 5E5 IU / mL were used, and various formulations in Example 1 were used to perform RNA extraction experiments respectively.
[0065] The extraction methods corresponding to formula A and formula E are as follows:
[0066] a. Take 1.5mL centrifuge tubes and mark them separately, and add 10 μL of nucleic acid extraction reagent 1 to each tube;
[0067] b. Add 200 μL of the sample to be tested in each tube, cover the tube cap, shake and mix for 5 seconds, and centrifuge briefly;
[0068] c. Add 600 μL of nucleic acid extraction reagent 2 and 5 μL of nucleic acid extraction reagent 3 to each tube, cover the tube, shake and mix for 10 seconds, and let stand at room temperature for 10 minutes;
[0069] d. Instant centrifugation, place the centrifuge tube on a magnetic separator, and slowly suck out the solution after 3 minutes (be careful not to touch the brown matter ...
Embodiment 3
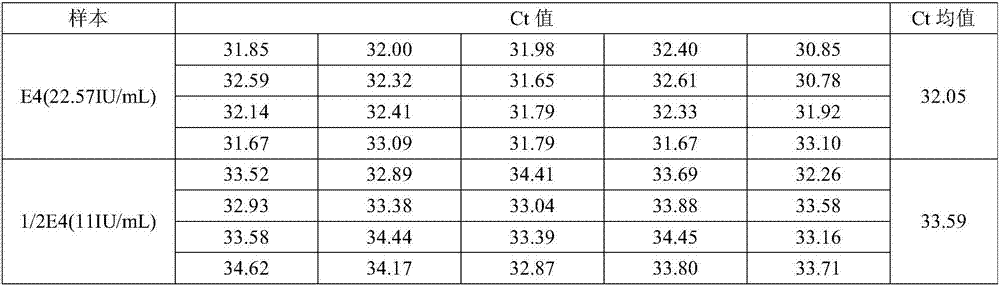
[0081] Extraction sensitivity and stability testing
[0082] The HCV-positive plasma sample E1 was selected, and a part thereof was taken out in triplicate for 10-fold dilution to obtain 10-fold diluted samples E2, E3, and E4, respectively.
[0083] The nucleic acid concentrations of samples E1, E2, E3 and E4 were measured using the formula B (Sunshine Bio HCV Nucleic Acid Quantitative Detection Kit) described in Example 1 to obtain the data in Table 2.
[0084] Table 2: Formulation B measured values
[0085]
E1
E2
E3
E4
Ct value
29.75
33.11
35.95
40
Concentration (IU / mL)
2.55E+04
2.66E+03
393.43
22.57
[0086] According to Table 2, it can be seen that the concentration calculated by formula B measuring sample E4 is 22.57IU / mL, which is lower than the lower sensitivity limit of formula B 25IU / mL.
[0087] Dilute the E4 sample 2-fold to obtain 1 / 2 E4 sample with a theoretical concentration of 11IU / mL. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com