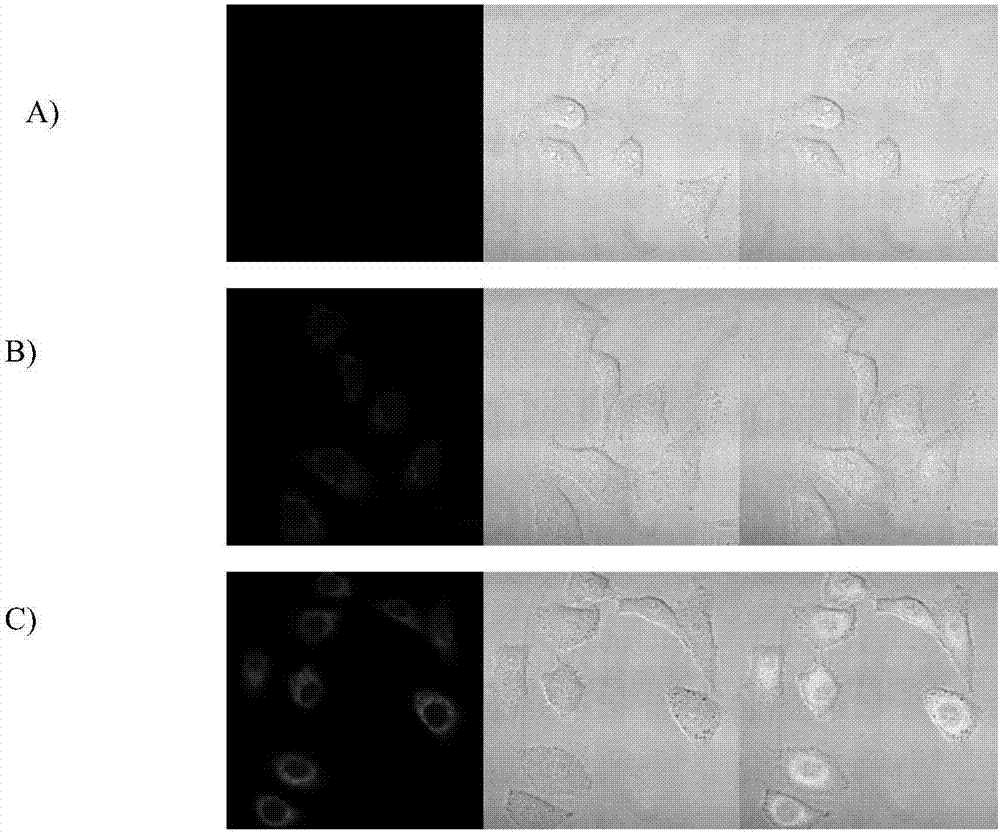
Light-sensitive targeted anti-tumor predrug for killing tumor cells in response to hydrogen peroxide as well as preparation method and application of light-sensitive targeted anti-tumor predrug
A hydrogen peroxide, tumor cell technology, applied in anti-tumor drugs, chemical instruments and methods, pharmaceutical formulations, etc., can solve the problems of limited clinical application of anti-tumor, low treatment efficiency, poor selectivity, etc., to improve adverse pharmacokinetics. The effect of dynamics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The synthesis of embodiment 1 prodrug (CM-1)
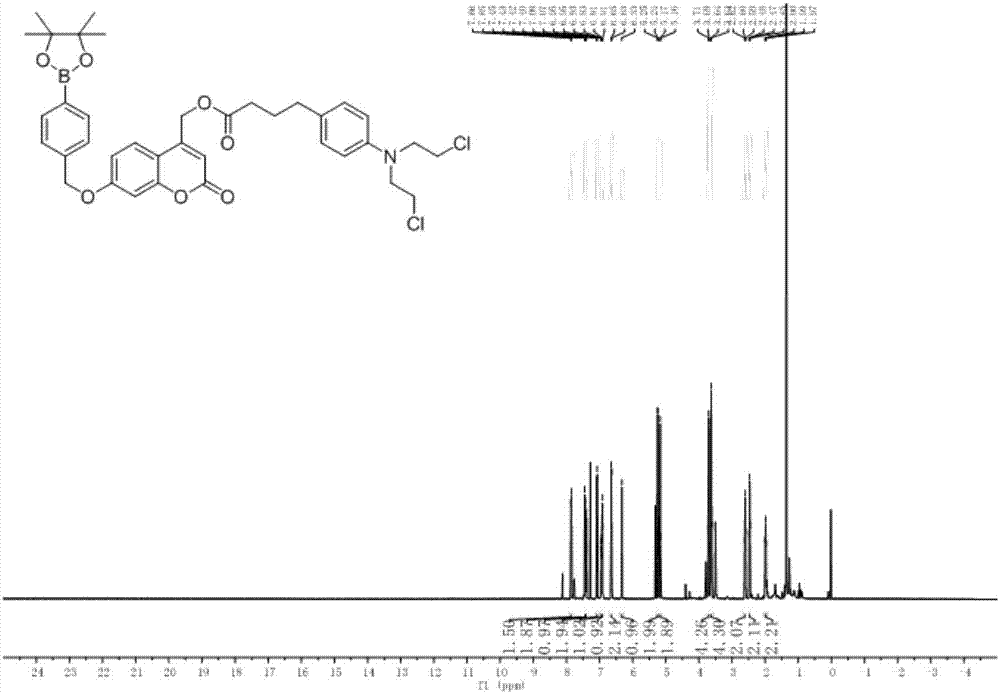
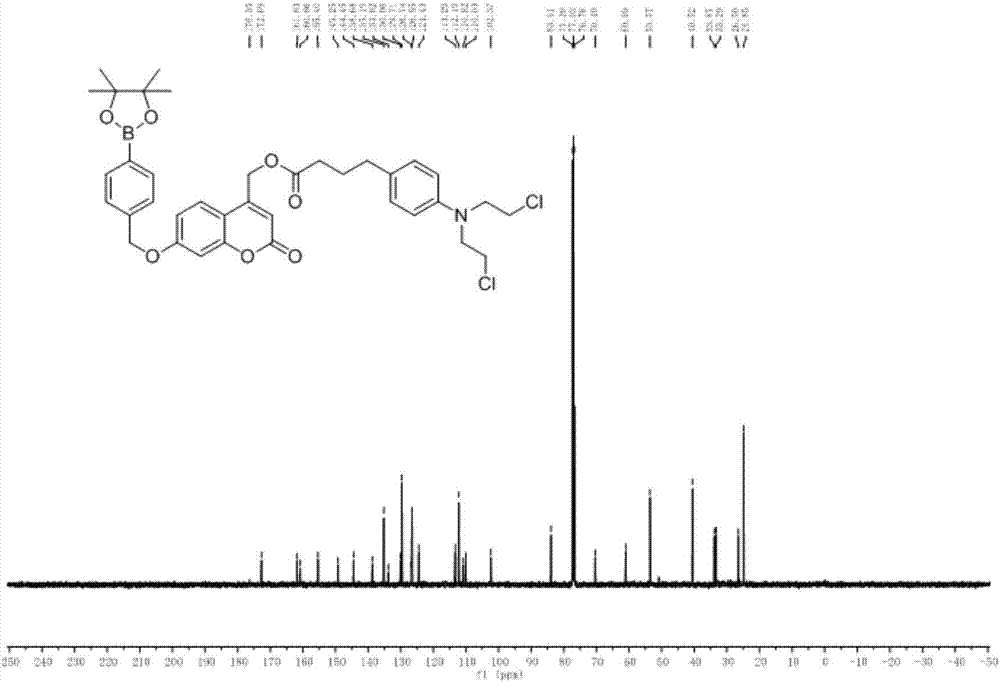
[0042] Compound 1-3 was put into a three-necked flask containing DCM, and after it was completely dissolved, DMAP (1.5 equivalents) and DCC (2.5 equivalents) were added successively. After activation for 10 min, 2.2 equivalents of chlorambucil was dissolved in 10 mL of DCM and Pour into the bottle, the whole reaction needs N 2 Protected and reacted overnight. Add 50mL DCM to the bottle, wash with water (7×50mL), wash with saturated sodium chloride (2×50mL), dry over anhydrous sodium sulfate, filter, and rotary evaporate to remove the organic solvent to obtain the crude product, which is separated by thin-layer chromatography to obtain the product For CM-1, the developer used was DCM(D) / MeOH(M)=15:1, and the yield was 83%. H NMR spectrum see figure 1 , see C NMR figure 2 .
[0043] 1 H NMR (500MHz, CDCl 3)δ7.85(d,J=8.1Hz,3H),7.42(dd,J=16.4,8.4Hz,6H),7.08(d,J=8.7Hz,4H),6.97–6.88(m,4H), 6.68–6.60(m,4H),6.33(s,2H),5.25...
Embodiment 2
[0044] The synthesis of embodiment 2 prodrugs (CM-1)
[0045] Compound 1-3 was put into a three-necked flask containing DCM. After it was completely dissolved, DMAP (0.1 equivalent) and DCC (1 equivalent) were added successively. After activation for 10 min, 1 equivalent of chlorambucil was dissolved in 10 mL of DCM and Pour into the bottle, the whole reaction needs N 2 Protected and reacted overnight. Add 50mL DCM to the bottle, wash with water (7×50mL), wash with saturated sodium chloride (2×50mL), dry over anhydrous sodium sulfate, filter, and rotary evaporate to remove the organic solvent to obtain the crude product, which is separated by thin-layer chromatography to obtain the product For CM-1, the developer used was DCM(D) / MeOH(M)=15:1, and the yield was 61%.
Embodiment 3
[0047] Compound 1-2 (100 mg) was added to a round bottom flask containing 10 mL of DMF, dissolved completely, and 1.2 equivalents of potassium carbonate solid was added, and 1.2 equivalents of 4-bromomethylbenzene was dissolved in 10 mL of DMF, and slowly added dropwise to the flask , react at room temperature for 2h. After the reaction, add 20mL DCM to the bottle, wash with water (7×50mL), take the organic phase and wash with saturated sodium chloride (2×50mL), dry over anhydrous sodium sulfate, filter, and remove the organic solvent by rotary evaporation to obtain the crude product. Compound 2 was separated by thin-layer chromatography, the developer used was D / M=10:1, and the yield was 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com