Escherichia coli soluble expression vector capable of efficiently obtaining recombinant protein
A technology for expressing vectors and recombinant proteins, applied in the direction of vectors, nucleic acid vectors, viruses/phages, etc., can solve the problems of low expression of foreign proteins, many operation steps, and low purity, and achieve high application value, simplified operation steps, The effect of improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
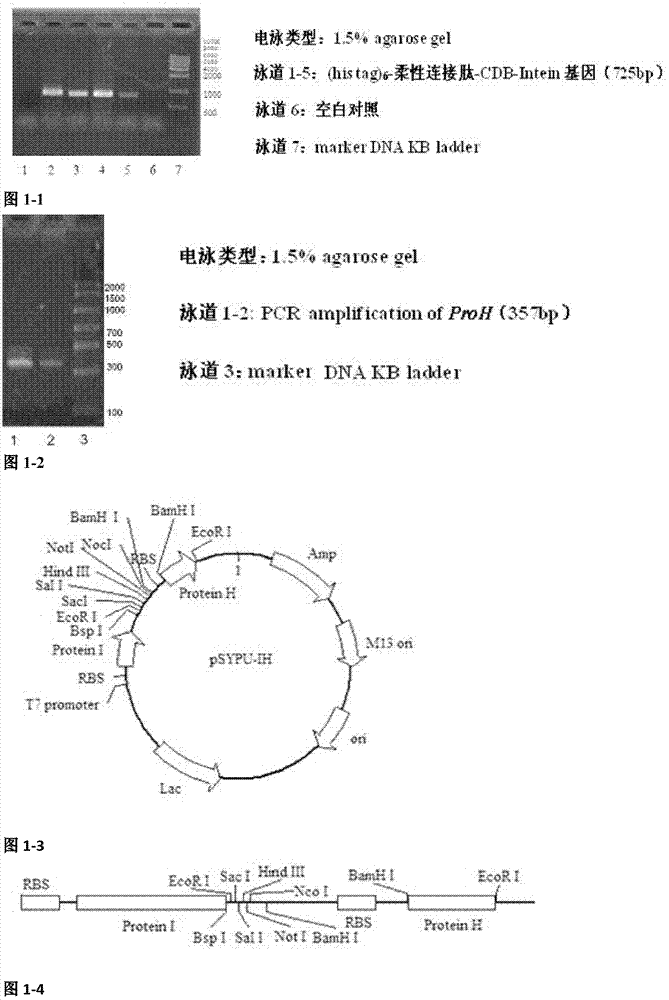
[0031] Embodiment 1: soluble expression vector pSYPU-IH constructs
[0032] Strain: E.coli BL21(DE3) is preserved in our laboratory
[0033] Primers:
[0034] LG4S-F1: 5'-GGTGGCGGCGGTAGTGGCGGCGGTGGTAGTAAAATCGAAGAAGGTAAACTGACAAATCC-3'
[0035] LG4S-F2: 5'-CGCCATATG(CAT) 5 CACGGTGGCGGCGGTAGTGGCGGCGGTGGTAGT-3'
[0036] LG4S-R1: 5'-CGCGGATCCGAATTCGAGCTCGCTCTTCCGTTGTGTACAATGAT-3'
[0037] ProH-F1: 5'-CGCGAATTCTAAGAAGGAGATATACATATGAGCGATAAAATTATTCACCTGAC-3'
[0038] ProH-R1: 5'-ACTACCACCGCCGCCACTACCGCCGCCACCGCTGCTGGCCAGGTTAGC-3'
[0039] ProH-F2: 5'-GGTGGCGGCGGTAGTGGCGGCGGTGGTAGTATGAGCGATAAAATTATTCACCTGAC-3'
[0040] ProH-R2: 5'-CGCGGATCCTTAGCTATGATGATGATGATGGTGGCTGCTGGCCAGGTTAG-3'
[0041] ProH-F: 5'-CGCGAATTCTAAGAAGGAGATATACATATGAGCGATAAAATTATTCACCTGAC-3'
[0042] ProH-R: 5'-CGCGGATCCTTAGCTATGATGATGATGATGGTGGCTGCTGGCCAGGTTAG-3'
[0043] ANGP-F1: 5'-GGTGGTTGCTCTTCCAACGATGGATATATAAGAGGAAGTAACG-3'
[0044] ANGP-R1: 5'-CGCGAATTCTTACTTTTTGCCACCGCATGTATTACT-3'
[0045] 1.1 (h...
Embodiment 2
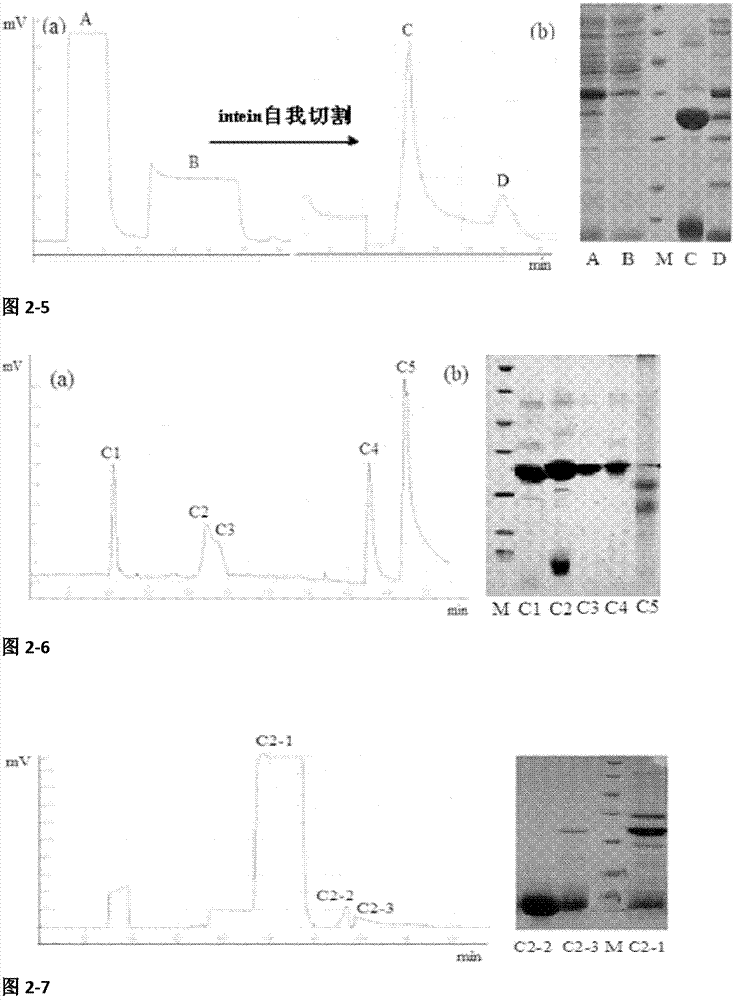
[0062] Example 2: Soluble expression, separation and purification of recombinant scorpion venom active peptide ANGP
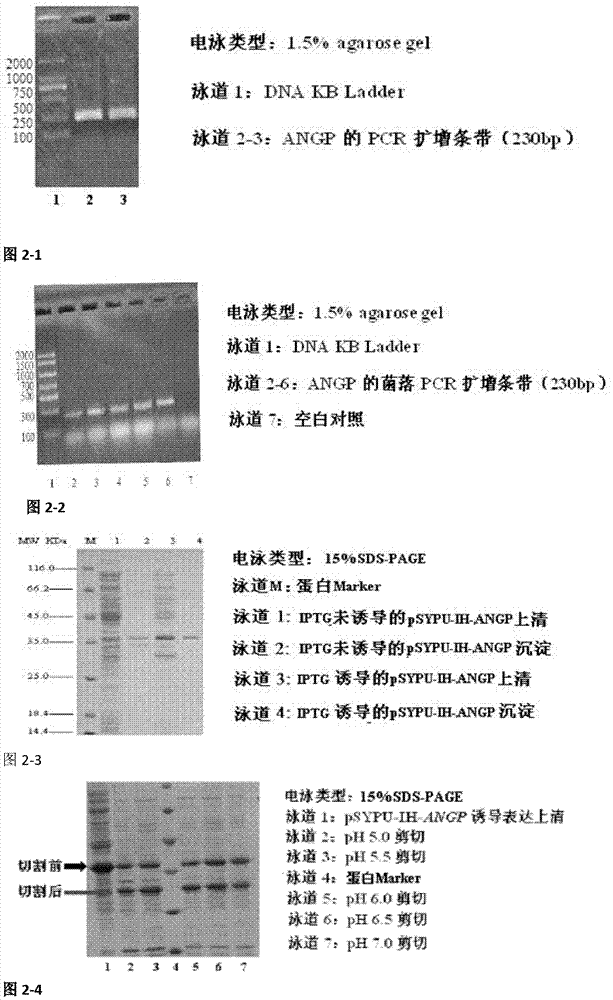
[0063] 2.1 Construction of recombinant plasmid pSYPU-IH-ANGP
[0064] Using pET28a-ANGP as a template, with Bsp I and EcoR I restriction endonuclease recognition site ANGP F / R as primers, PCR amplification of ANGP gene (see appendix) Figure 2-1 ). The ANGP gene was digested with EcoR I at 37°C for 2 hr, separated by 1.5% agarose gel electrophoresis, and the ANGP gene was recovered by gel (see appendix). Figure 2-2 ), the recovered product of ANGP gene after being digested by EcoR I single enzyme was subjected to Bsp I single enzyme digestion, and the Bsp I single enzyme digestion of ANGP gene was performed at 50°C for 1 hr. The recombinants were screened by LB-Amp and verified by sequencing.
[0065] 2.2 Induction and expression of recombinant plasmid pSYPU-IH-ANGP
[0066] Take the recombinant plasmid pSYPU-IH-ANGP, heat-transform E. coli BL21 (DE3) comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com