Application of vanillin in the preparation of medicines for treating/preventing bone metabolic diseases
A technology of vanillin and bone metabolism, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
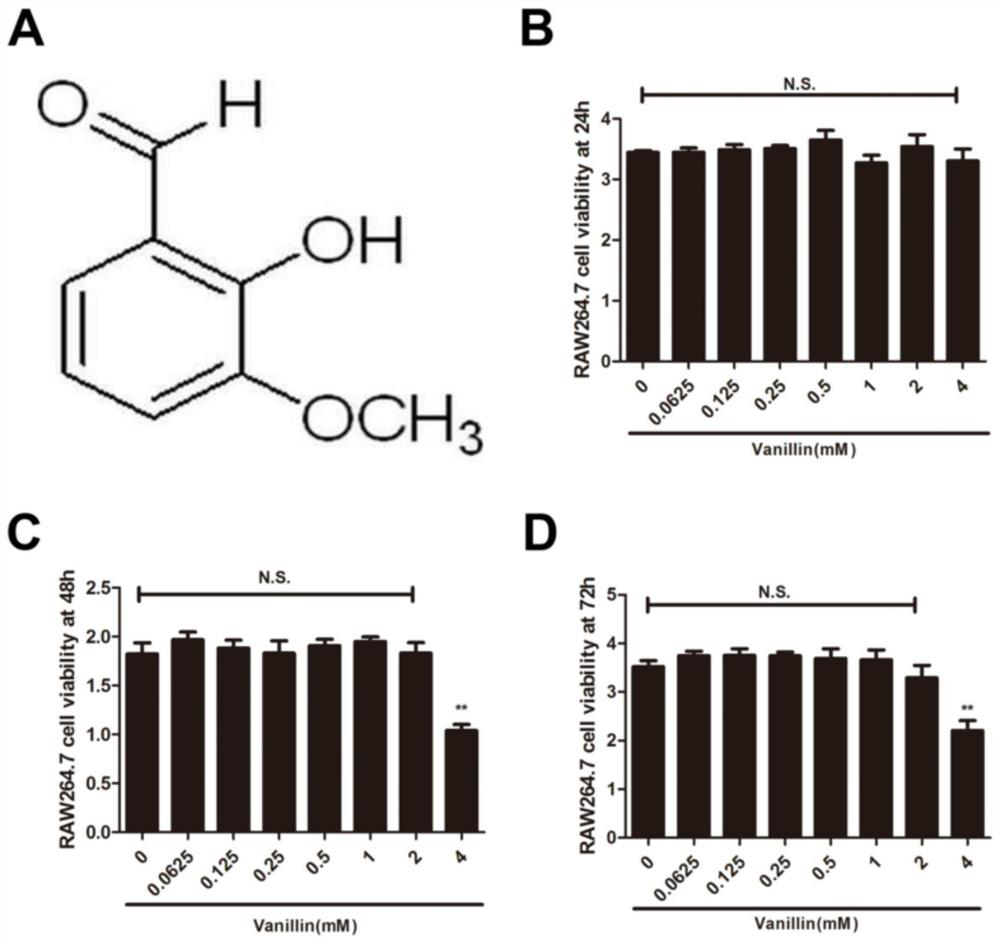
[0031] Example 1. Effect of vanillin concentration on inhibiting the proliferation of osteoclast precursors
[0032] Divide RAW264.7 cells to 1 x 10 3Cells / well were inoculated into 96-well plates, and the medium was discarded after the cells were full. The medium was composed of: DMEM high-glucose medium + 10% fetal bovine serum + 1% double antibody (penicillin) at the final concentration Add 0 mM, 0.0625 mM, 0.125 mM, 0.25 mM, 0.5 mM, 1 mM, 2 mM, 4 mM vanillin to each well plate containing fresh medium (the concentration of DMSO is controlled at less than 0.1%) to interfere with RAW264.7 cells . After culturing for 24h, 48h and 72h respectively, the cell viability was detected by the CCK-8 method, and the effect of the proliferation of RAW264.7 cells under the intervention of vanillin was observed. A step-by-step experimental investigation.
[0033] The result is as figure 1 shown, where figure 1 A is the chemical formula of vanillin; figure 1 In the middle, B-D are th...
Embodiment 2
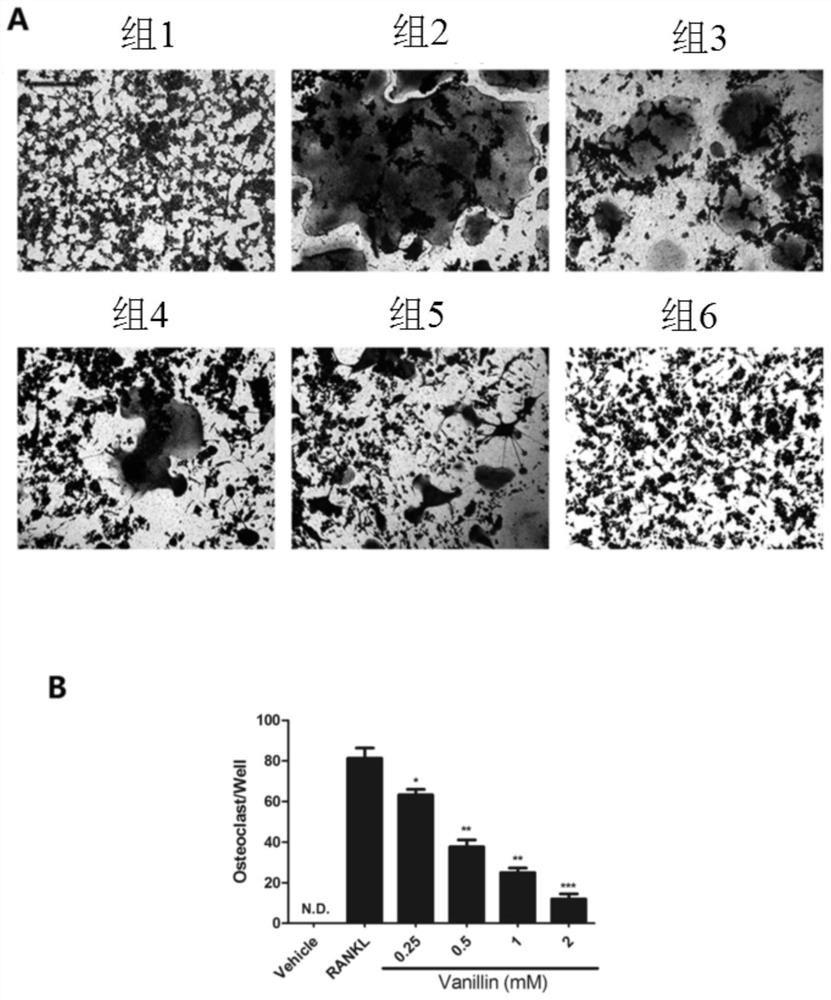
[0035] Example 2. Vanillin concentration-dependent reduction in RANKL-induced osteoclast formation (TRAP positive cells)
[0036] Taking no RANKL induction as the control group, RANKL induction and the effect of different vanillin concentrations as the experimental group, the groups were grouped according to the following conditions:
[0037] Group 1: no RANKL induction, 0mM vanillin control group;
[0038] Group 2: RANKL (50ng / mL) induction, 0mM vanillin experimental group;
[0039] Group 3: RANKL (50ng / mL) induction, 0.25mM vanillin experimental group;
[0040] Group 4: RANKL (50ng / mL) induction, 0.5mM vanillin experimental group;
[0041] Group 5: RANKL (50ng / mL) induction, 1mM vanillin experimental group;
[0042] Group 6: RANKL (50ng / mL) induction, 2mM vanillin experimental group.
[0043] Divide RAW264.7 cells into 2 x 10 3 Cells / well were inoculated into 96-well plates, and the medium was discarded after the cells were full. The medium was composed of: DMEM high-gl...
Embodiment 3
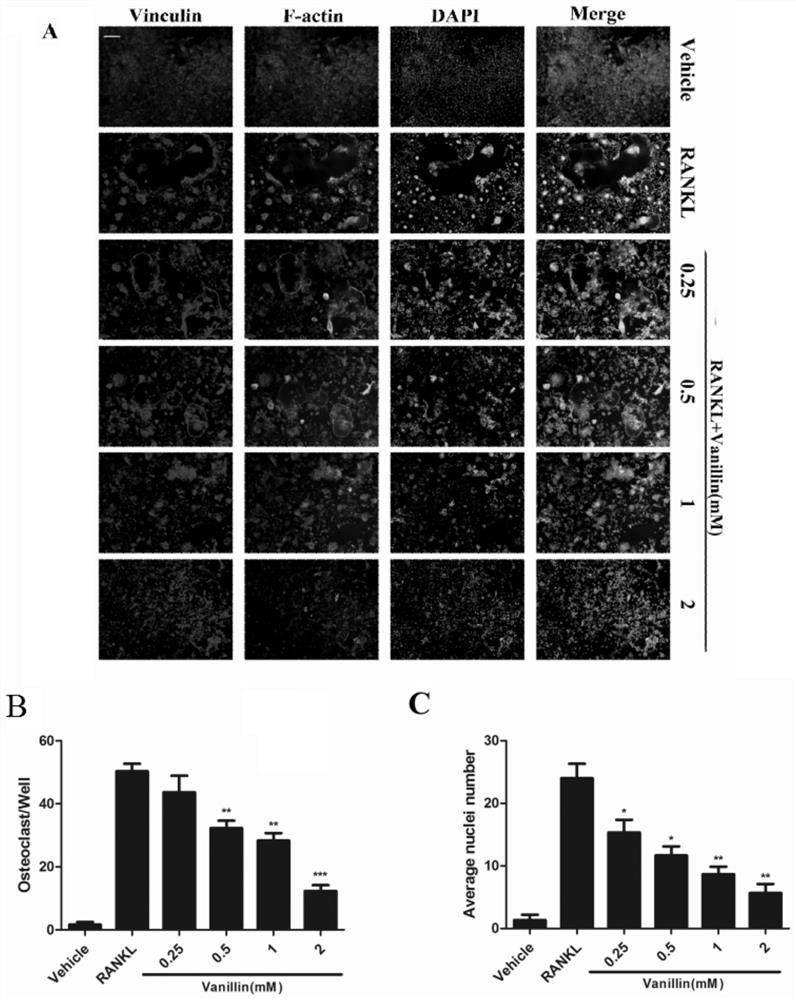
[0045] Example 3. Vanillin concentration-dependent reduction of RANKL-induced multinucleated mature osteoclast formation
[0046] Taking no RANKL induction as the control group, RANKL induction and the effect of different vanillin concentrations as the experimental group, the groups were grouped according to the following conditions:
[0047] Group 1: no RANKL induction, 0mM vanillin control group;
[0048] Group 2: RANKL (50ng / mL) induction, 0mM vanillin experimental group;
[0049] Group 3: RANKL (50ng / mL) induction, 0.25mM vanillin experimental group;
[0050] Group 4: RANKL (50ng / mL) induction, 0.5mM vanillin experimental group;
[0051] Group 5: RANKL (50ng / mL) induction, 1mM vanillin experimental group;
[0052] Group 6: RANKL (50ng / mL) induction, 2mM vanillin experimental group.
[0053] The RAW264.7 passaged cells were continued to be cultured, and after the cell density at the bottom of the flask reached 90%, the cells were scraped with a cell scraper to collect t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com