Solid dispersoid of selexipag and pharmaceutic adjuvant and preparation method of solid dispersoid
A technology of solid dispersion and pharmaceutical excipients, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, drug combinations, etc., can solve the problems affecting drug bioavailability and low solubility, and achieve fast dispersion and dissolution, Good solubility and easy-to-achieve effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
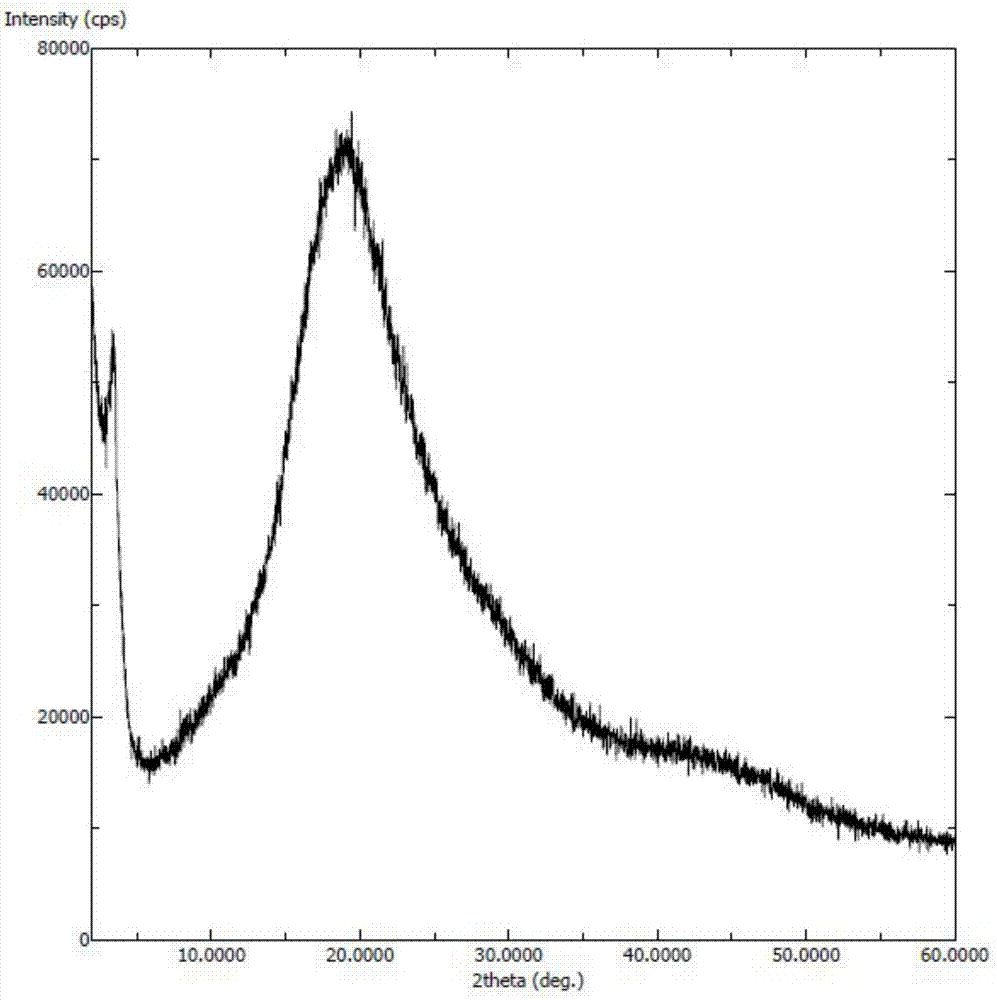
[0048] Dissolve Selexipa (50 mg) in isopropanol (600 microliters) and water (900 microliters), heat to 60°C and stir to dissolve. The above solution was rapidly cooled to -10°C, and a white solid was precipitated, filtered, and dried to obtain amorphous seraxipa, and the X-ray powder diffraction pattern was as follows: figure 1 As shown, there is no characteristic peak of the crystal form of Selesipah in the X-ray powder diffraction pattern.
Embodiment 2
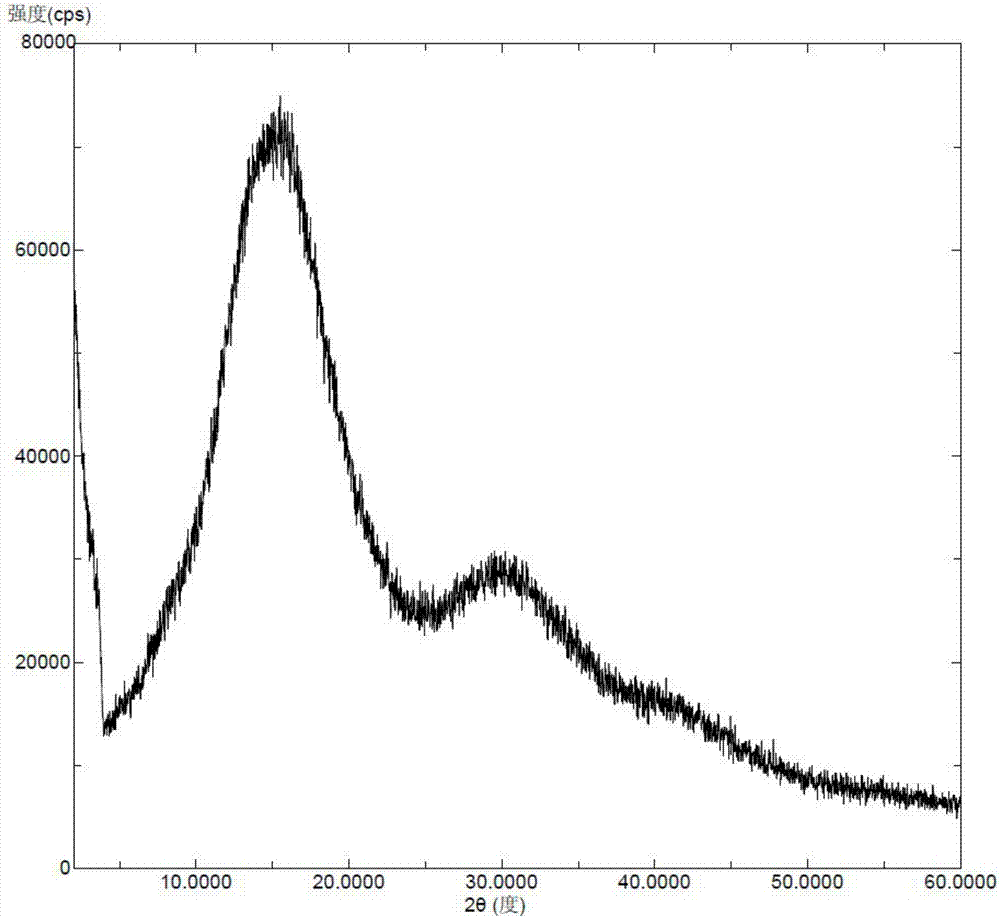
[0050] Dissolve Selexipah (50 mg) in ethanol (600 microliters) and water (600 microliters), and stir to mix evenly at 40°C. The above solution was slowly concentrated to dryness in a rotary evaporator to obtain a white solid, which gave amorphous selexipa, and the X-ray powder diffraction pattern was as follows: figure 2 As shown, there is no characteristic peak of the crystal form of Selesipah in the X-ray powder diffraction pattern.
Embodiment 3
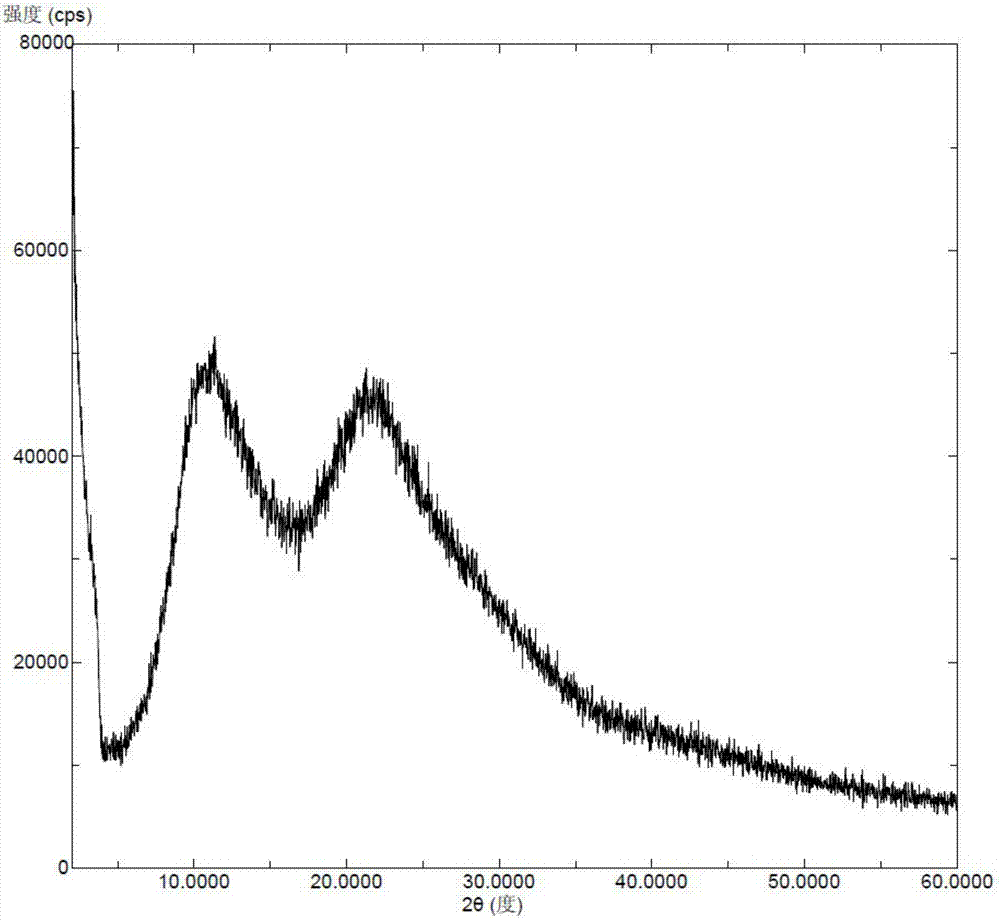
[0052] Add Selexipa (5 g) and povidone K30 (10 g) into water (300 ml), heat to 60° C. and stir to dissolve. The above solution was dried with a JISL micro-spray dryer LSD-48, and the inlet temperature was maintained at 60°C and the outlet temperature was 50°C. The outlet material was collected to obtain a white solid, which was further vacuum-dried to obtain a mixture of amorphous Selexipah and povidone-K30. Solid dispersion. X-ray powder diffraction pattern as image 3 As shown, in the X-ray powder diffraction pattern of the solid dispersion, there is no characteristic peak of the crystalline form of Selexipah after deducting the background peaks of the pharmaceutical excipients.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com