Targeting EGFR and HER2 double-specific antibody and application thereof
A bispecific antibody and antibody technology, applied in the direction of application, antibody, specific peptide, etc., can solve the problems of easy tolerance and inability to exert therapeutic effect for a long time, and achieve the effect of reducing the amount of antibody, having a significant killing effect, and a remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
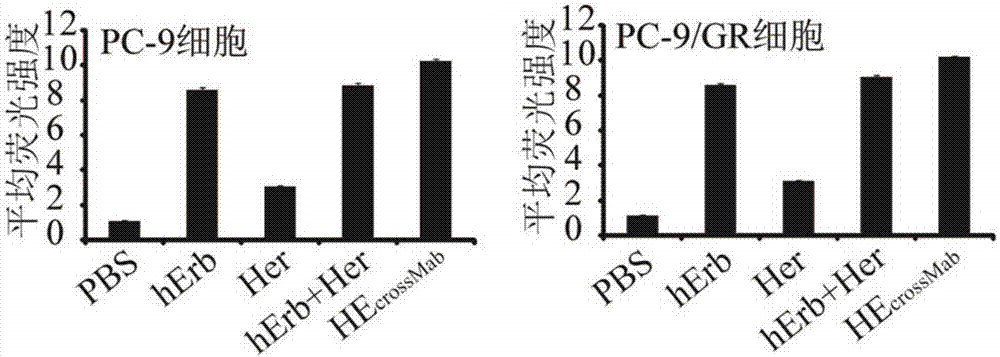
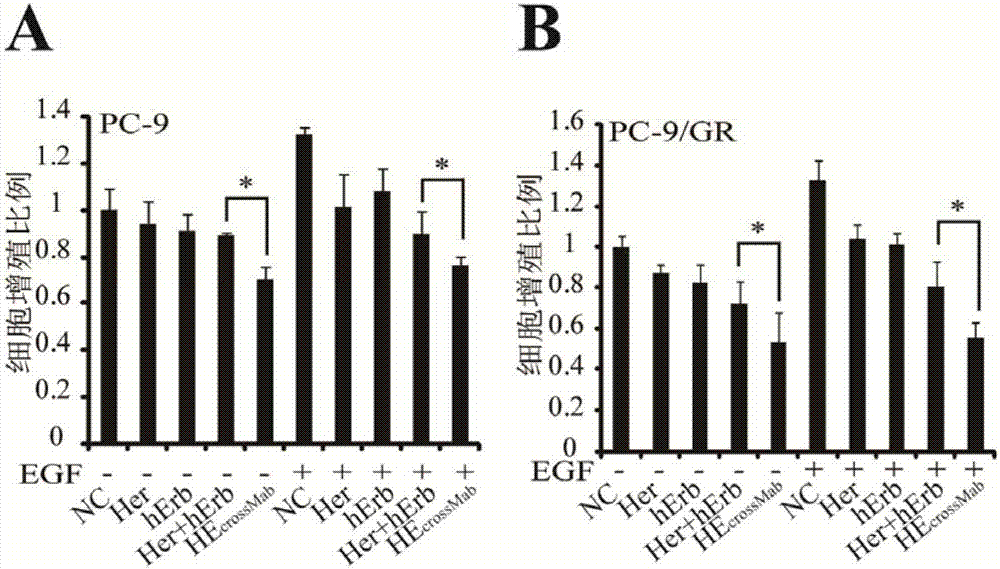
Examples
Embodiment 1
[0038] Embodiment 1, recombinant plasmid construction
[0039] All experimental operations in plasmid construction are referred to "Molecular Cloning Experiment Guide" (Second Edition, Science Press). The DNA molecules shown in the sequences 1, 3, 5 and 7 of the sequence listing were respectively inserted into the pZJC vector to obtain 4 kinds of recombinant plasmids. Using the heavy and light chains of the humanized cetuximab antibody and Herceptinumab as templates, the corresponding site mutations and fragment swaps were introduced, and primers were designed for amplification. The corresponding primers are shown in Table 1 below:
[0040] Table 1 Synthetic Crossmab structural gene primer sequence
[0041]
Embodiment 2
[0042] Example 2, preparation of bispecific antibodies
[0043] (1) Culture well-growing HEK293F cells into 125ml shake flasks, and adjust the number of cells to 5×10 the day before transfection 5 / ml, the activity reaches more than 90%, and the medium is 30ml. Cultivate at 37°C and 125rpm.
[0044] (2) Measure the cell number and viability on the day of transfection, and the viability should be greater than 95%. Replace with fresh Freestyle293F medium after centrifugation. Take 30μg combined plasmid DNA in Opti-MEM medium, the total volume is 1ml.
[0045] (3) Add 60 μl of 293F liposomes into Opti-MEM medium, the total volume is 1 ml.
[0046] (4) Incubate at room temperature for 5 minutes, slowly add DNA into the diluted liposomes, gently blow and mix with a pipette tip, and let stand at room temperature for 20-30 minutes. After incubation, mix 2ml of liposomes and plasmids solution was added to the cell suspension.
[0047] (5) Continue to culture in a shaker at 37° C...
Embodiment 3
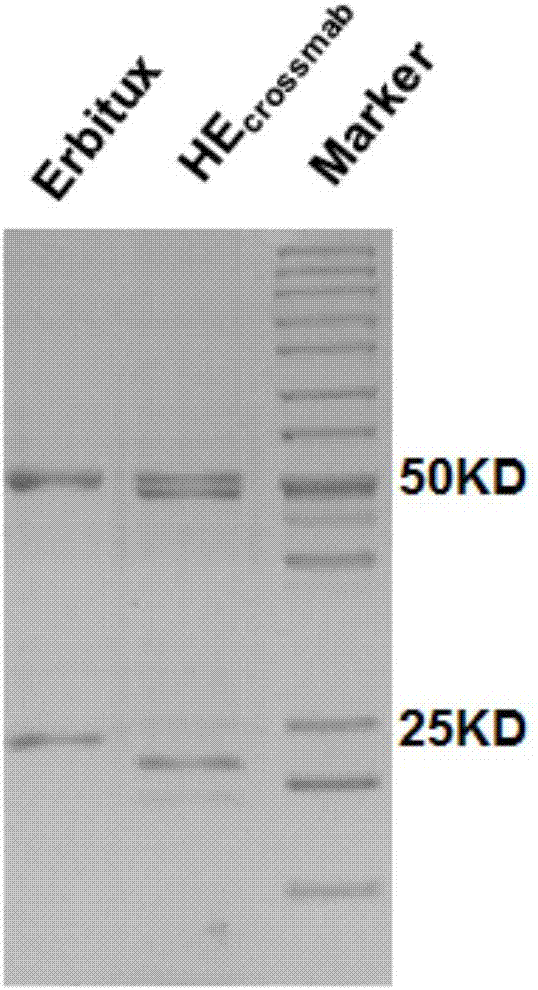
[0054] Embodiment 3, SDS-PAGE detection
[0055] (1) Sample treatment: add three times the volume of the sample to one volume of 4×Loading Buffer (Loading Buffer is divided into denatured reduction and denatured non-reduced types), mix well, boil for 10 min, centrifuge at 12000 rpm for 10 min, and set aside.
[0056] (2) Glue compounding: First prepare the separation gel, add isopropanol on top of the separation gel, let it stand at room temperature for about 30 minutes until the gel solidifies, and pour off the isopropanol. Then prepare concentrated gel, add the concentrated gel and insert it into the comb mold quickly, let it stand at room temperature for 30 minutes until the gel solidifies, and pull out the comb mold.
[0057] (3) Sample loading: Use a small pipette tip to draw the sample for sample loading, load 5 μl of the sample on the Marker, and supplement the gel holes on both sides with 1×LoadingBuffer.
[0058] (4) Electrophoresis: The protein electrophoresis buffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com