SCR denitration catalyst and preparation method thereof
A denitration catalyst and catalyst technology, which are used in catalyst activation/preparation, chemical instruments and methods, chemical elements of heterogeneous catalysts, etc., can solve the problems of narrow activity temperature window and poor low temperature activity, and achieve wide denitration temperature window, good Effect of medium and low temperature denitration activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
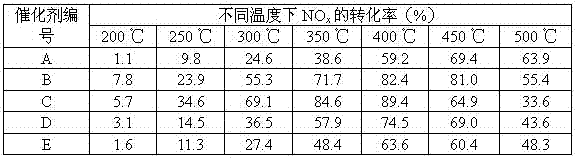
Examples
Embodiment 1
[0019] Step 1, make soluble cerium salt, soluble tungsten salt and soluble titanium salt into an aqueous solution according to the molar ratio of cerium, tungsten and titanium elements at 20:10:100, and add urea to control urea / (Ce+W+Ti) The molar ratio was 5.0.
[0020] Step 2: Put the mixed solution obtained in Step 1 in a water bath, and conduct a hydrothermal treatment at a water bath temperature of 90°C for 10 hours to promote the complete precipitation of cerium, tungsten, and titanium ions.
[0021] In step 3, the hydrothermal precipitate obtained in step 2 was filtered, washed, dried at 105 °C for 30 min, and then calcined in a muffle furnace at 550 °C for 5 h under air atmosphere to obtain catalyst A.
Embodiment 2
[0023] Step 1, soluble cerium salt, soluble tungsten salt and soluble titanium salt are made into aqueous solution according to the molar ratio of cerium element, tungsten element and titanium element at 20:10:100, and add 1ml H 2 o 2 solution (1 mol / L), control H 2 o 2 The molar ratio of titanium ions to the solution is 10:100; urea is finally added to control the molar ratio of urea / (Ce+W+Ti) to 5.0.
[0024] Step 2: Put the mixed solution obtained in Step 1 in a water bath, and conduct a hydrothermal treatment at a water bath temperature of 90°C for 10 hours to promote the complete precipitation of cerium, tungsten, and titanium ions.
[0025] In step 3, the hydrothermal precipitate obtained in step 2 was filtered, washed, dried at 105 °C for 30 min, and then calcined in a muffle furnace at 550 °C for 5 h under air atmosphere to obtain catalyst B.
Embodiment 3
[0027] Step 1, make soluble cerium salt, soluble tungsten salt and soluble titanium salt into an aqueous solution according to the molar ratio of cerium, tungsten and titanium elements at 20:10:100, and add 2 ml H 2 o 2 solution (1 mol / L), control H 2 o 2 The molar ratio of titanium ions to the solution is 20:100; urea is finally added to control the molar ratio of urea / (Ce+W+Ti) to 5.0.
[0028] Step 2: Put the mixed solution obtained in Step 1 in a water bath, and conduct a hydrothermal treatment at a water bath temperature of 90°C for 10 hours to promote the complete precipitation of cerium, tungsten, and titanium ions.
[0029] In step 3, the hydrothermal precipitate obtained in step 2 was filtered, washed, dried at 105 °C for 30 min, and then calcined in a muffle furnace at 550 °C for 5 h in an air atmosphere to obtain catalyst C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com