Random copolymer polypropylene catalyst and preparation method thereof
A polypropylene catalyst and random copolymerization technology, applied in the field of polypropylene, can solve the problems of low activity and low catalyst orientation ability, and achieve the effect of high random phase content, high ethylene content and increasing ethylene copolymerization ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] Preparation method of a part of internal electron donor
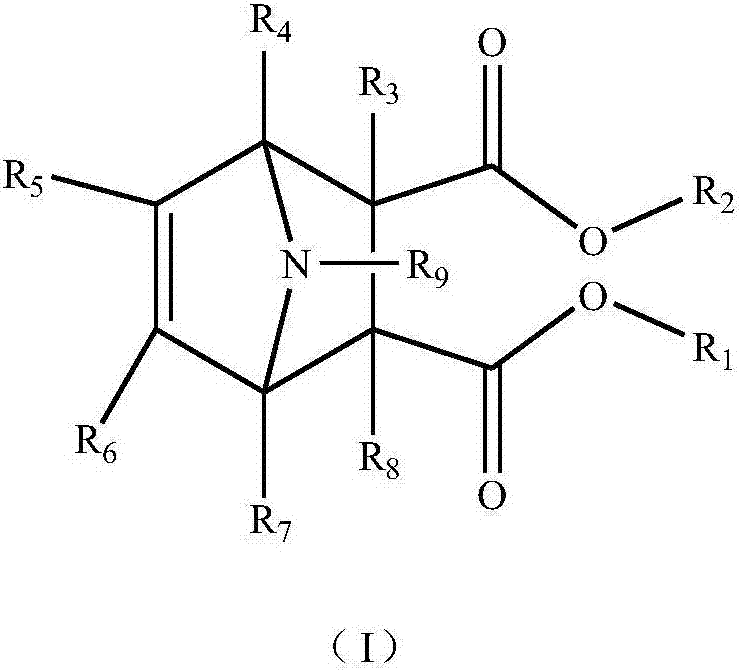
[0055] (1) Dimethyl N-methyl-7-azabicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate
[0056] In a 250ml round bottom flask, add 8.1g (0.1mol) N-methylpyrrole and 14.4g (0.1mol) dimethyl maleate, 100mL solvent toluene, install a reflux condenser, and heat to reflux under magnetic stirring for 8 Hour. Pure crystallization after completion of the reaction, the product obtained is a white solid with a yield of 83%. 1H NMR (400MHz, CDCl3): 2.27(3H), 3.08(2H), 3.56(2H), 3.67(6H), 5.59(2H), MS(EI) m / z: 225(M+).
[0057] (2) Dicyclopentyl 1-chloro-N-methyl-7-azabicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate
[0058] Add 11.5g (0.1mol) 1-chloro-N-cyclopentylpyrrole and 25.2g (0.1mol) dicyclopentyl maleate in a 250ml round bottom flask, 100mL solvent toluene, a reflux condenser tube, The reaction was heated under reflux for 10 hours under magnetic stirring. Pure crystallization after completion of the reaction, the product obtai...
Embodiment 1
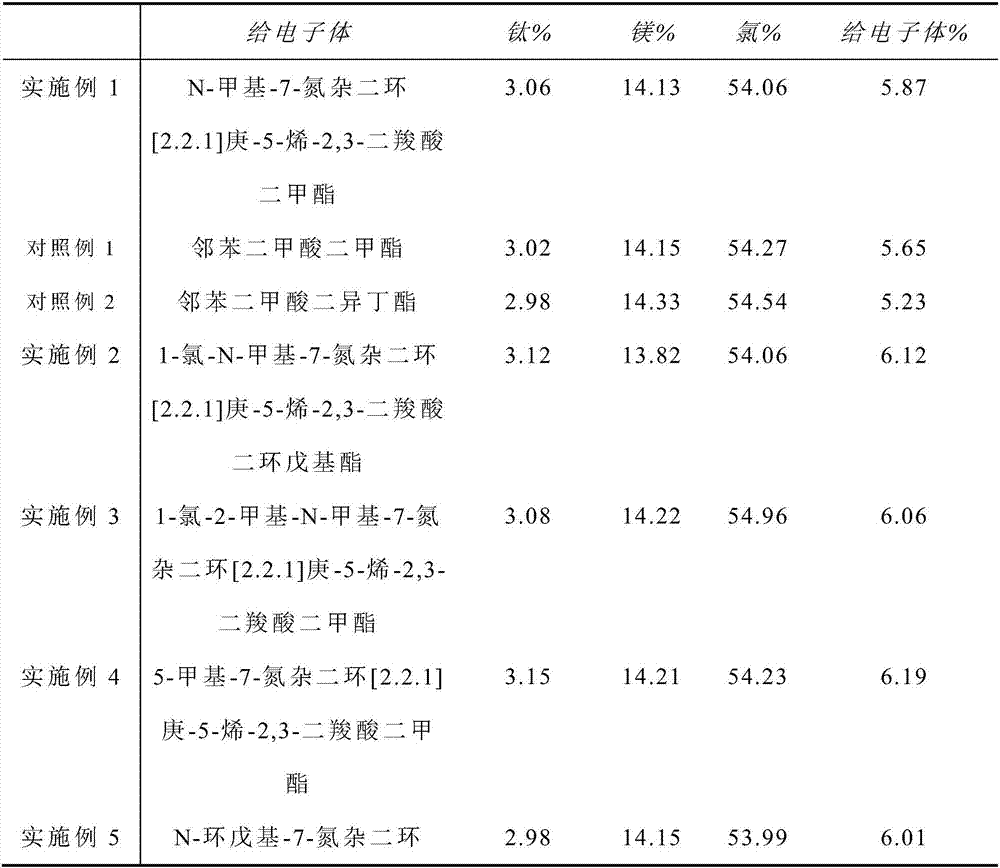
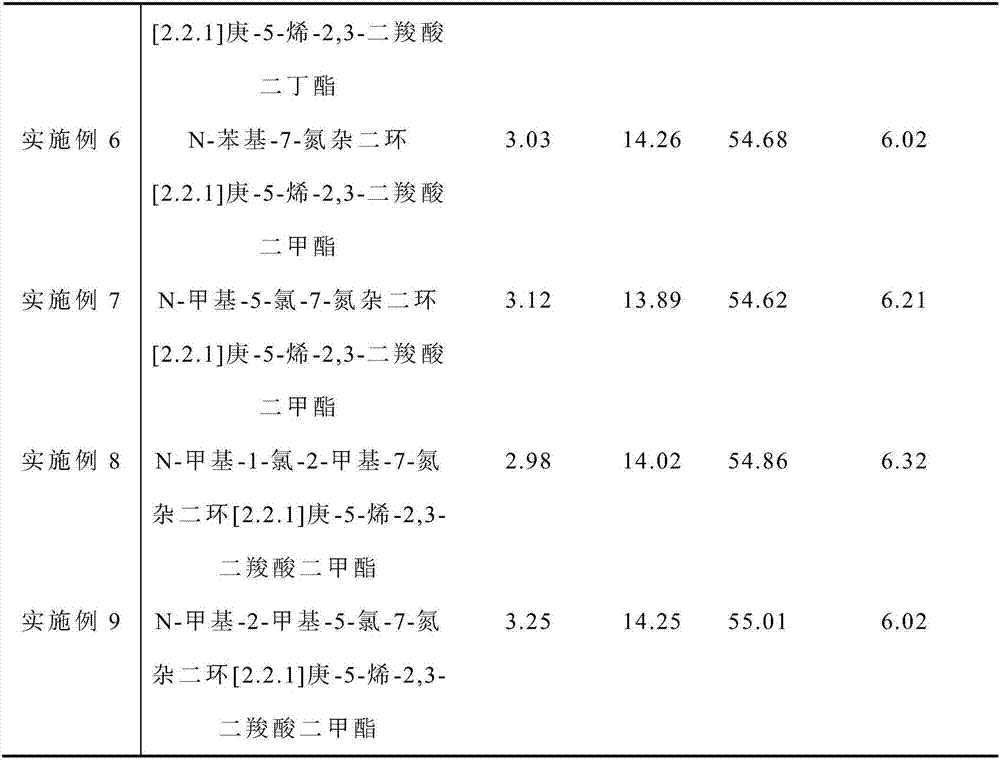
[0063] Under anhydrous and oxygen-free conditions, 5.0 grams of microspherical magnesium chloride alcoholate particles (self-made, the preparation steps are the same as the document CN1110281A, the average particle size is 50 μm, the specific surface is 150-300m / g, and the molar ratio of alcohol and magnesium chloride content is 2.76 : 1, molecular formula: MgCl 2 ·2.76CH 3 CH 2 OH) was added to 30 ml of titanium tetrachloride liquid at -20°C, and after 1 hour of reaction, the temperature was gradually raised to 60°C; 0.82g of N-methyl-7-azabicyclo[2.2.1]hept-5 Dimethyl-ene-2,3-dicarboxylate, gradually heated to 120°C, reacted for 2 hours, and filtered; then added 30 ml of titanium tetrachloride, reacted at 120°C for 1 hour, and filtered. Wash with 20 ml of hexane at 60° C. for 5 times, wash once with 10 ml of hexane at room temperature, and dry in vacuum to obtain a magnesium chloride-supported catalyst.
[0064] The content of each component in the catalyst is shown in ...
Embodiment 2~9
[0072] Preparation of supported catalyst: In addition to the internal electron donor compound, 1-chloro-N-methyl-7-azabicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic acid dicyclopenta Dimethyl 1-chloro-2-methyl-N-methyl-7-azabicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate, 5-methyl- Dimethyl 7-azabicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate, N-cyclopentyl-7-azabicyclo[2.2.1]hept-5-ene -Dibutyl 2,3-dicarboxylate, N-phenyl-7-azabicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate dimethyl, N-methyl- Dimethyl 5-chloro-7-azabicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate, N-methyl-1-chloro-2-methyl-7-aza Dimethyl bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate, N-methyl-2-methyl-5-chloro-7-azabicyclo[2.2.1]heptane -5-ene-2,3-dicarboxylic acid dimethyl ester, all the other are the same as embodiment 1.
[0073] The content of each component in the catalyst is shown in Table 1.
[0074] The content of each component in the catalyst of table 1 is shown in the table
[0075]
[0076]
[0077] three-catalys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com