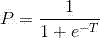Gene marker and detection method for detecting benign and malignant liver tumors, and kit
A gene marker, liver tumor technology, applied in the direction of biochemical equipment and methods, microbial determination/examination, etc., can solve the problems of patients' mental burden, patient suffering, diagnosis difficulties, etc., and achieve easy dynamic monitoring and good user experience. , high sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. Screening of Liver Tumor Gene Markers
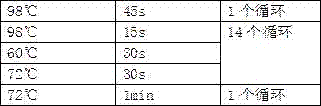
[0027] 10 ng of plasma DNA was extracted from samples from 20 patients with liver cancer and 20 patients with benign liver tumors. This step can be carried out using any methods and reagents suitable for extracting plasma DNA well known to those skilled in the art.
[0028] 2) End-fill the plasma DNA, overhang A and connect with the sequencing adapter:
[0029] Prepare a reaction mixture containing 50uL plasma DNA, 7uL End Repair & A-Tailing Buffer and 3uL End Repair & A-Tailing Enzyme mix according to the Kapa Hyper Perp Kit instructions (the total volume is 60uL), incubate at 20°C for 30 minutes, and then incubate Incubate at 65°C for 30 minutes. Prepare the following ligation reaction mixture in a 1.5mL low adsorption EP tube: 5uL Nuclease free water, 30uL Ligation Buffer and 10 uL DNA Ligase. Add 5uL of sequencing adapters to 45uL ligation reaction mixture, mix, heat at 20°C for 2...
Embodiment 2
[0048] Example 2. Effectiveness of Liver Tumor Gene Markers
[0049] This example verifies the effectiveness of the liver tumor gene markers of the present invention for distinguishing benign from malignant liver tumors.
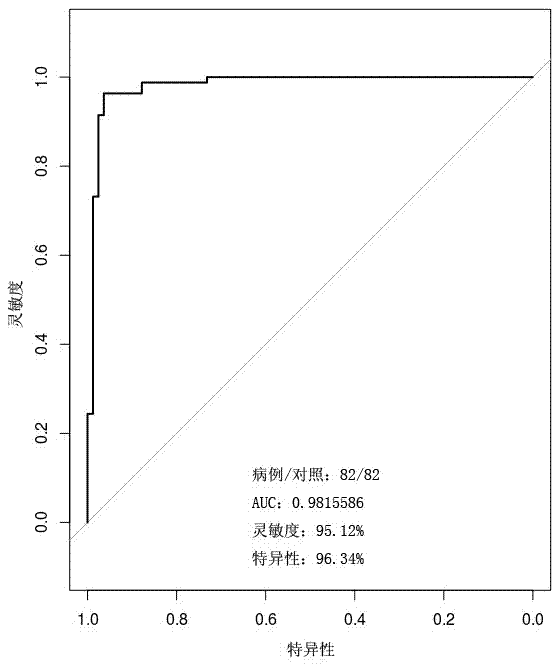
[0050] According to the method of Example 1, the 5-hmC content of the 10 liver cancer gene markers described in the present invention in the first batch of 164 samples (82 cases of liver cancer and 82 cases of benign liver tumors) was determined.
[0051]The standardized 5-hmC content of each gene marker is multiplied by the corresponding weighting coefficient of the marker in Example 1 to obtain the predictor t of the gene marker, and then the predictor t of each gene marker is added to obtain The total predictor T, and then the total predictor T is transformed according to the following formula to obtain the score P:
[0052]
[0053] If P>0.5, the subject sample suffers from liver cancer; if P≤0.5, the subject suffers from benign liver tumor.
[0054...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com