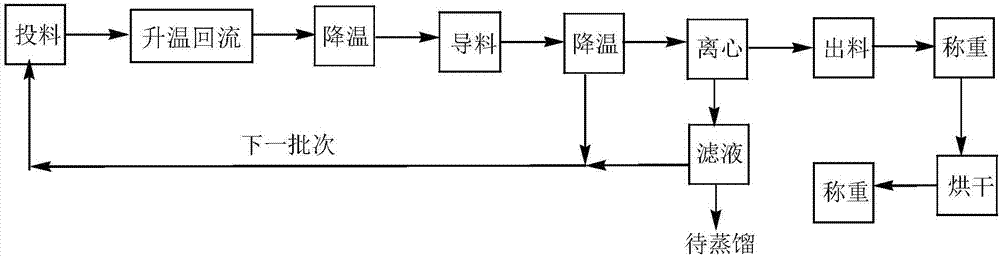
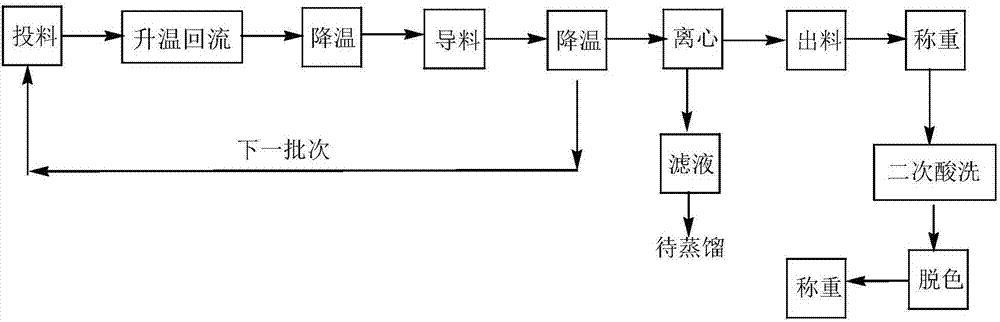
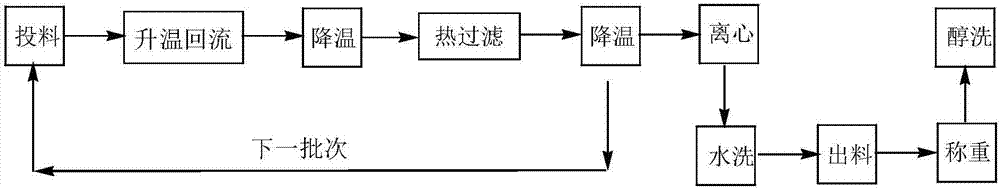
Preparation method of N-lauroyl-L-lysine
A technology of lauroyl lysine and lysine, which is applied in the field of preparation of N-lauroyl-L-lysine, can solve the problems of many side reactions, low yield, and inability to achieve high yield and high yield. The effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0048] In order to facilitate understanding of the present invention, the present invention enumerates the following examples. Those skilled in the art should understand that the examples are only used to help understand the present invention, and should not be regarded as specific limitations on the present invention.
[0049] equipment used
[0050] The equipment used in the following examples is shown in Table 1 below.
[0051] Table 1
[0052]
[0053]
[0054] The materials used are as follows:
[0055] Lauric acid: scientific name dodecanoic acid, molecular formula: CH 3 (CH 2 ) 10COOH, molecular weight: 200. It has the smell of laurel oil, white needle-like crystals. The relative density is 0.8679 (50°C). The melting point is 44°C. The boiling point is 225°C (13.3 kPa, 100 mm Hg). Insoluble in water, soluble in ethanol, ether and benzene. Used in the production of alkyd resins, wetting agents, detergents, insecticides, etc. Separated from fatty acids i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com