Application of benzothiazole substituted benzofuran quinoline derivatives in the preparation of anti-drug-resistant bacteria drugs
A technology of benzofuranquinoline and benzothiazole, which is applied in the directions of antibacterial drugs, resistance to vector-borne diseases, pharmaceutical formulations, etc., can solve problems such as no discovery, and achieve the effect of low toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only some, not all, embodiments of the present invention. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention. Embodiment 1 antibacterial activity screening
[0033] The minimum inhibitory concentration MIC (μg / mL) value of benzothiazole substituted benzofuran quinoline derivatives was determined by micro broth dilution method.
[0034] 1. Test compounds are shown in Table 1, and their preparation methods have been disclosed in the following documents:
[0035] Wang Zhengya, benzothiazole substituted benzofuran quinoline derivatives as G-quadruplex DNA fluorescent ligan...
Embodiment 2
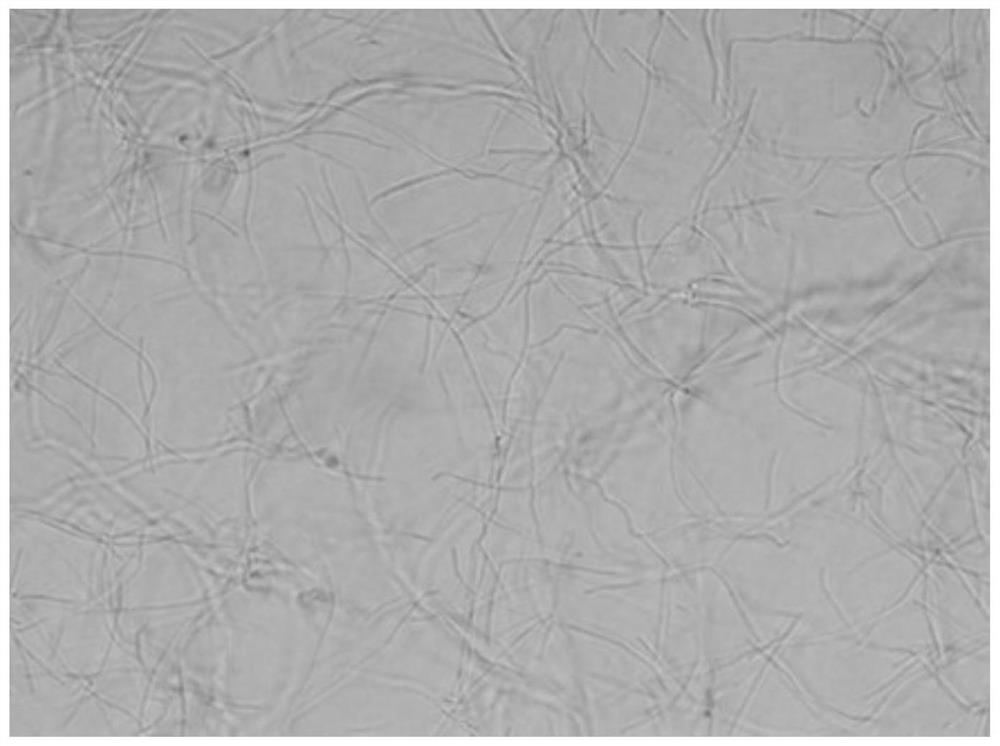
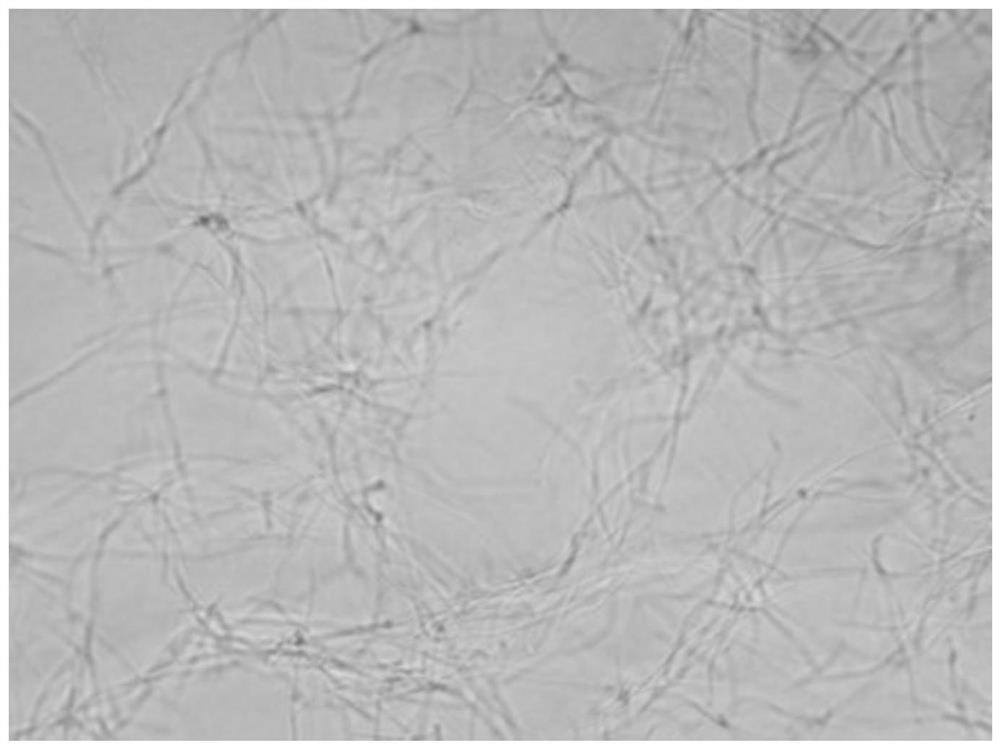
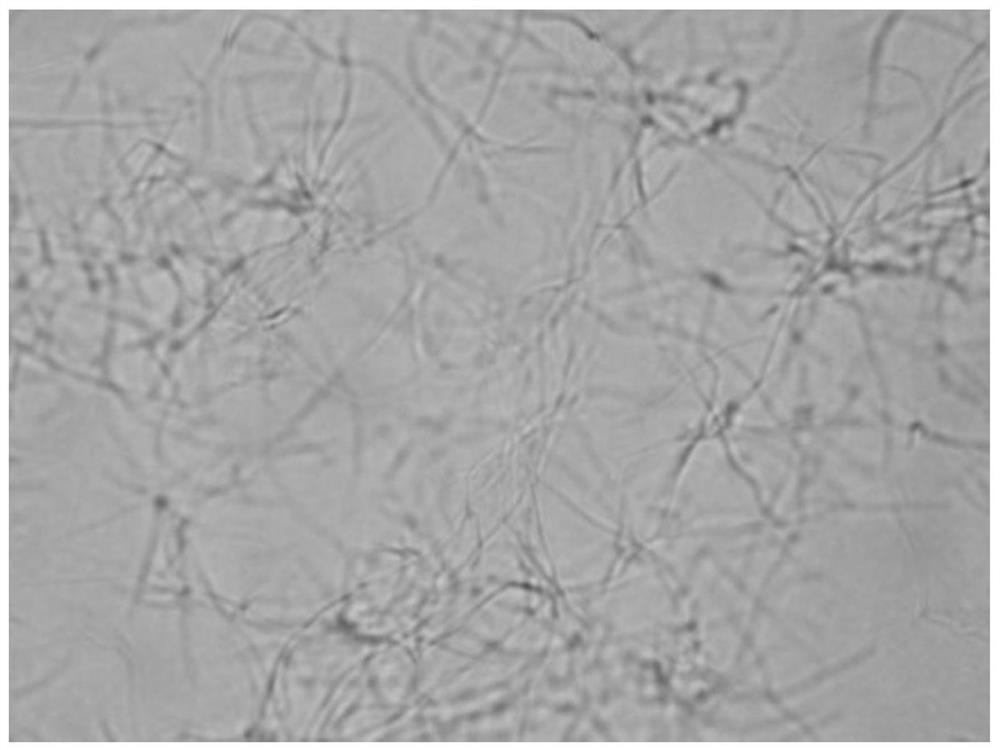
[0047] The growth form of Bacillus subtilis under the action of benzothiazole substituted benzofuran quinoline derivatives was observed by microscope:
[0048] 1. The tested compounds are shown in Table 1, and Bacillus subtilis B.Subtilis 168 was selected as the indicator bacterium.
[0049] 2. The prepared MH broth medium was sterilized by high-pressure steam for 30 minutes, and cooled; the MH broth was diluted to a concentration equivalent to 0.5 McFarland turbidimetric tube (bacteria content 0.5×10 8 each / mL), add 150 μ L of bacterial solution to each hole in the 96-well plate; then, add the stock solution of the compounds 1 to 6 prepared in Example 1 respectively in the 96-well plate, so that the concentration of each test compound They are the half-value of MIC in Table 2; the blank control group uses DMSO.
[0050] Figure 1 to Figure 7In order to detect the results, compared with the blank group, the bacteria in the drug-dosed group were significantly extended, and it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com