Method for preparing indole compound and application thereof
An aniline compound and compound technology are applied in the field of organic synthesis, can solve the problems of cumbersome steps and high production costs, and achieve the effects of simple steps, convenient operation and reasonable route design.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The embodiment of the present invention provides a preparation method of indole compounds, which comprises:
[0017] S1. Reacting the aniline compound and ethylene oxide under the action of the first catalyst to obtain N-hydroxyethylaniline compound.
[0018] Aniline compounds are a common class of chemical raw materials. They come from a wide range of sources and are cheap and easy to obtain. Using them as raw materials can achieve the purpose of reducing costs. The oxygen in ethylene oxide has a strong electron-pulling effect, which makes the two carbons in ethylene oxide show a certain degree of electropositivity, and the ring tension of the three-membered ring makes ethylene oxide easy to be The nucleophile attacks and opens the ring. The aniline compound is reacted with oxirane, and the amino group of the aniline compound acts as a nucleophilic group to attack the oxirane to open the ring to form an N-hydroxyethylaniline compound. Among them, the structural formu...
Embodiment 1
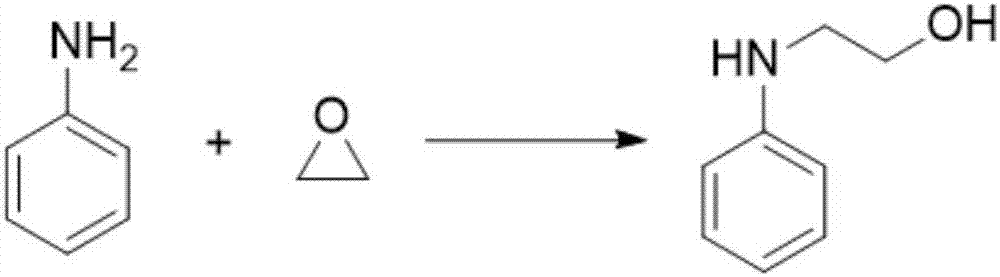
[0031] This embodiment provides a kind of N-hydroxyethylaniline compound, and its reaction formula is
[0032]
[0033] Its preparation method is:
[0034] Weigh 10mmol of aniline and 0.1mmol of ZnCl in the reaction flask 2 , dissolved in 100ml of acetonitrile, and added 50mmol of ethylene oxide into the reaction flask at -20°C. Maintain -20°C and stir the reaction for 20h. After separation and purification, N-hydroxyethylaniline was obtained (yield 87.3%).
Embodiment 2
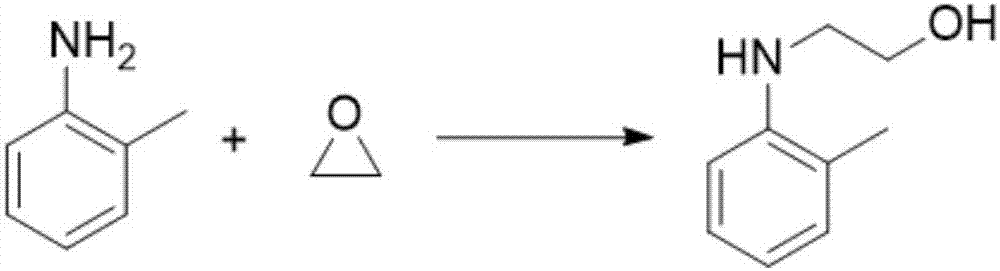
[0036] This embodiment provides a kind of N-hydroxyethylaniline compound, and its reaction formula is
[0037]
[0038] Its preparation method is:
[0039] Weigh 10mmol of 2-methylaniline and 0.2mmol of AlCl in the reaction flask 3 , dissolved in 100ml of tetrahydrofuran, at 0°C, add 30mmol of ethylene oxide into the reaction flask. Maintain 0°C and stir the reaction for 10h. After separation and purification, N-hydroxyethyl-2-methylaniline was obtained (yield 83.1%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com