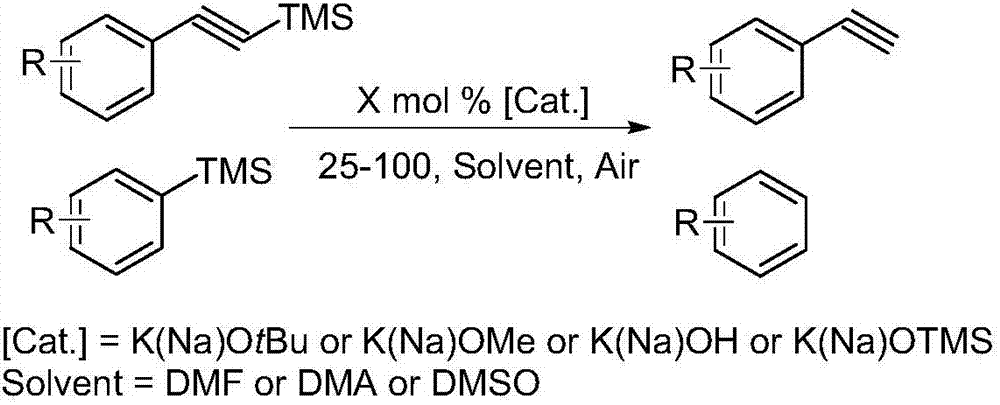
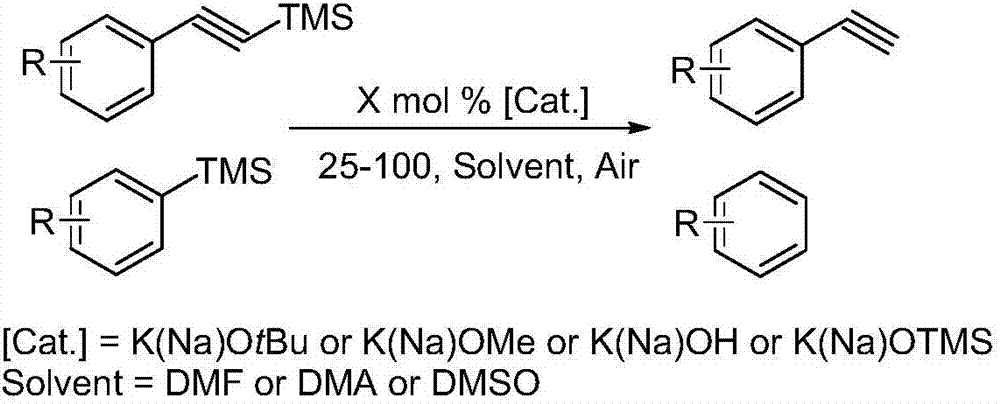
Practical novel method for nonmetal-catalyzed silicon disprotection
A non-metallic catalysis and deprotection technology, used in chemical instruments and methods, organic chemistry, hydrocarbons, etc., can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add trimethylphenylsilane (1 mmol), inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or potassium trimethylsilanolate (sodium) (0.05 mmol), 2 mL DMA or DMSO solvent to 10 mL In the sealed tube, heat and stir in an oil bath at 60°C for 6.5 hours, and track the reaction progress according to TLC. After the reaction is completed, add an equivalent amount of mesitylene or n-undecane to the crude product as an internal standard to measure by GC and GC-MS The exact yield of the product. According to GC and GC-MS, when using DMSO as reaction solvent, inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or trimethylsiliconate potassium (sodium) as catalyst, the yield of product is successively : 80%, 74%, 79%, 84%, 95%, 92%. When using DMA as the reaction solvent, the inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or potassium trimethylsiliconate (sodium) is used as the catalyst, and the...
Embodiment 2
[0020] 4-Methyltrimethylphenylsilane (1 mmol), inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or potassium trimethylsilanolate (sodium) (0.05 mmol), 2 mL DMA or DMSO solvent Add them to 10mL sealed tubes one by one, heat and stir in an oil bath at 60°C for 8 hours, and track the reaction progress according to TLC. GC-MS determined the exact yield of the product. According to GC and GC-MS, when using DMSO as reaction solvent, inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or trimethylsiliconate potassium (sodium) as catalyst, the yield of product is successively : 61%, 59%, 77%, 69%, 97%, 90%. When using DMA as reaction solvent, inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or potassium trimethylsiliconate (sodium) are as catalyst, and the yield of product is successively: 47%, 55%, 59%, 49%, 92%, 91%.
Embodiment 3
[0022] 3-Methyltrimethylphenylsilane (1 mmol), inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or potassium trimethylsilanolate (sodium) (0.05 mmol), 2 mL DMA or DMSO solvent Add them to 10mL sealed tubes one by one, heat and stir in an oil bath at 60°C for 8 hours, and track the reaction progress according to TLC. GC-MS determined the exact yield of the product. According to GC and GC-MS, when using DMSO as reaction solvent, inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or trimethylsiliconate potassium (sodium) as catalyst, the yield of product is successively : 67%, 52%, 71%, 59%, 90%, 83%. When using DMA as reaction solvent, inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or potassium trimethylsiliconate (sodium) are as catalyst, and the yield of product is successively: 51%, 52%, 54%, 55%, 89%, 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com