EGFR and CD47 targeting bispecific fusion protein, and preparation method and application thereof
A fusion protein and bispecific antibody technology, applied in the biological field, can solve the problems of inability to eliminate and kill tumor cells, macrophage incapacity, etc., and achieve the effect of enhancing anti-tumor immune response and good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Construction of novel bispecific antibody fusion protein Bi-SP
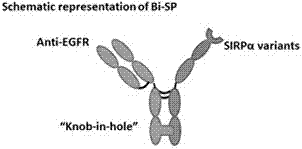
[0043] Using the "Knob-in-hole" antibody heavy chain constant region mutation technology, the Fc segment of the Pan side of the anti-EGFR and the Fc segment of the SIRPα mutant (SIRPα-Fc for short) are mutated, so that the Pan side of the anti-EGFR is compatible with CD47 One side of the receptor SIRPα mutant-Fc segment (SIRPα-Fc for short) can be correctly paired and assembled into the structure of the bispecific antibody fusion protein. The structure of the novel bispecific antibody fusion protein Bi-SP is as follows: figure 1 shown.
[0044] The Fc segment mutant Hole has the base sequence of SEQ ID NO:1 and the amino acid sequence described in EQ ID NO:2.
[0045] The Fc segment mutant Knobs has the base sequence of SEQ ID NO:3 and the amino acid sequence described in EQ ID NO:4.
[0046] The anti-EGFR Pan light chain of the mutation as described above has the base sequence of SEQ ID NO:5, ...
Embodiment 2
[0050] Example 2: Expression and purification of novel bispecific antibody fusion protein Bi-SP
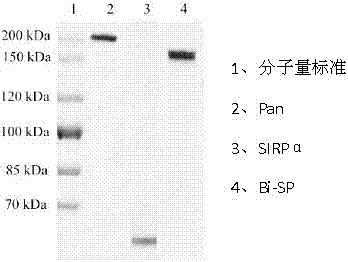
[0051] The verified correct cloned gene was connected to the expression vector, and then transformed into CHO cells for the expression of the bispecific antibody fusion protein. Using Dot Blotting, CHO cell monoclonals with higher expression levels can be screened, expanded, and the culture supernatant harvested. Using Protein A affinity chromatography technology, the bispecific soluble antibody fusion protein Bi-SP with a purity of more than 90% was purified. SDS-PAGE results see figure 2 , lane 1 is molecular weight standard, lane 2 is Pan, lane 3 is SIRPα mutant, and lane 4 is Bi-SP.
experiment example 1
[0052] Experimental Example 1: In vitro biological function research of Bi-SP
[0053] 1. Obtain lung cancer H292 cells (referred to as H292-R) and epidermal cancer A431 cells (referred to as A431-R) resistant to cetuximab
[0054] Cetuximab was added to the culture medium of H292 cells and A431 cells to a concentration of 1 μg / mL, and the culture was continued for 6 months. The successful cultivation of drug-resistant cell lines was verified by in vitro and in vivo growth inhibition tests.
[0055] 2. In vitro biological function research of Bi-SP
[0056] (1) The molecular weight and purity of Bi-SP were characterized by SDS-PAGE.
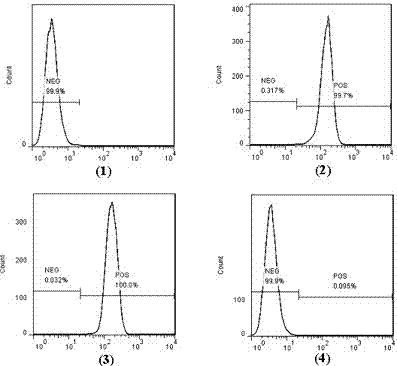
[0057] (2) Analyze the binding activity of Bi-SP to EGFR-positive 231 cells and CD47-positive HL-60 cells by flow cytometry (FACS) ( image 3 , Figure 4 ).
[0058] (3) The dissociation constant of Bi-SP was determined by Biacore analysis.
[0059] (4) The ability of Bi-SP to promote macrophage phagocytosis was determined by flow cytometry....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com