Preparation method of temperature response type nanoliposomes coated with matrix metalloproteinase inhibitors
A protease inhibitor and nano-liposome technology, applied in the field of nano-liposome and its preparation, can solve the problems of easy leakage of drugs and low stability, so as to improve anti-tumor ability, improve stability, and improve targeting sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] prescription:
[0034]
[0035] Preparation:
[0036] Add 3 mg of Marimastat to 3 mL of pH 6.5 phosphate buffer, sonicate until dissolved, and set aside. 51.6 mg of dipalmitoyl phosphatidyl choline, 6 mg of stearoyl lysolecithin, and 2.4 mg of polyethylene glycol material were added to 8 mL of a mixed solution of chloroform and methanol (volume ratio of 3:1), and sonicated for half a minute. After complete dissolution, put it on a rotary evaporator at 45°C and remove the organic solvent under reduced pressure, and then use the prepared 3mL Marimastat solution to hydrate for 1h at 45°C. The solution obtained after hydration was placed in a probe sonicator, and the power was 190W for 10 minutes. The ultrasonic mode was turned on for 3s and stopped for 4s. The solution obtained after sonication was extruded through a 0.22 μm filter to obtain the target solution.
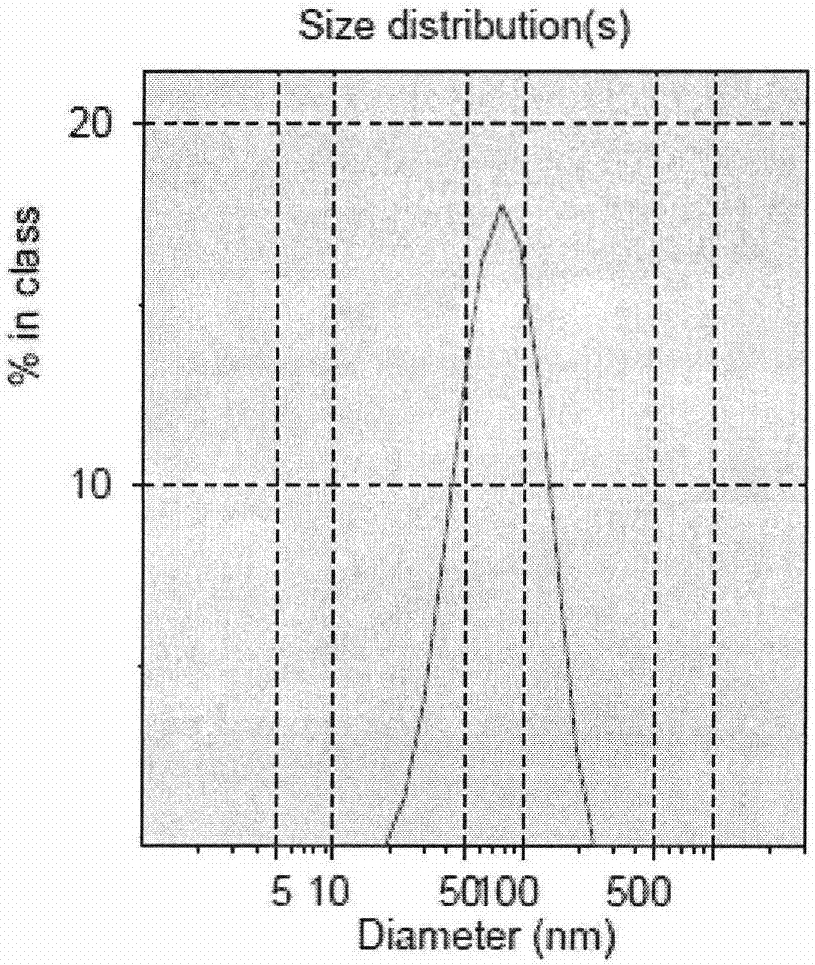
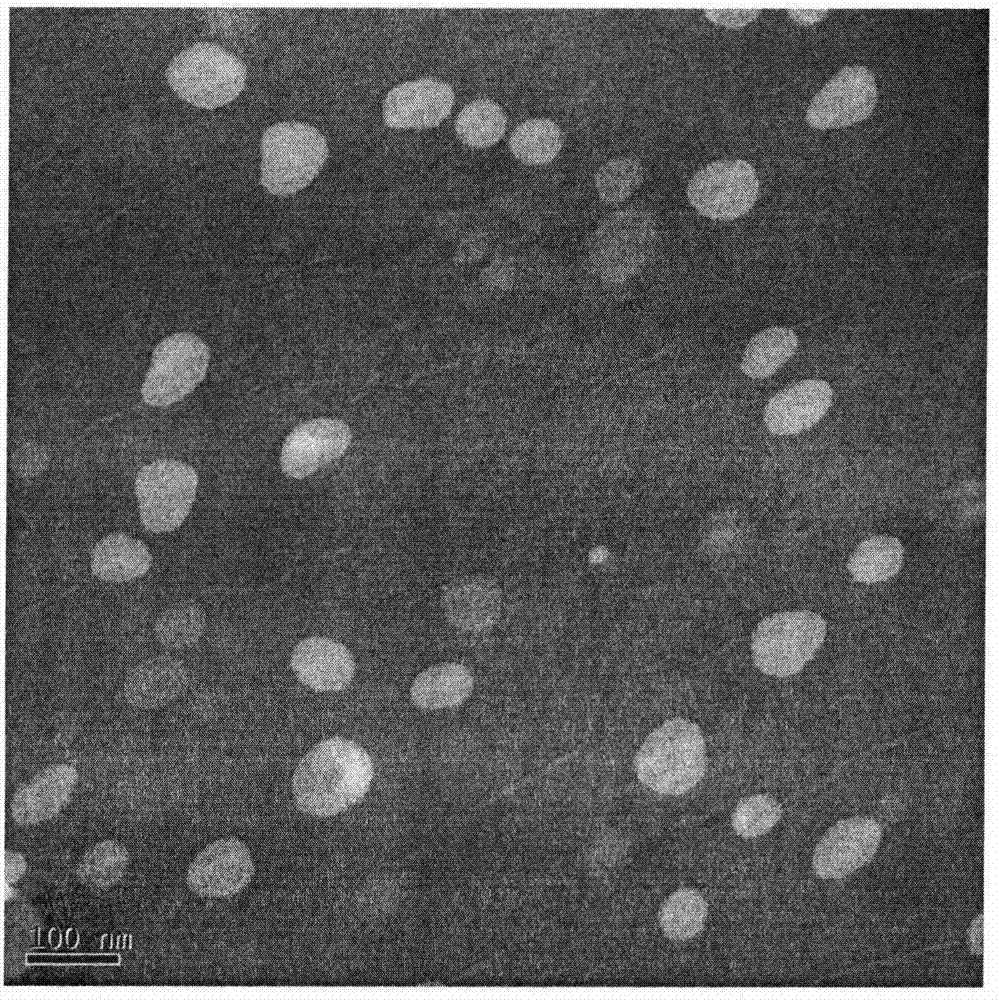
[0037] The particle size, polydispersity coefficient, and Zeta potential of the temperature-responsive n...
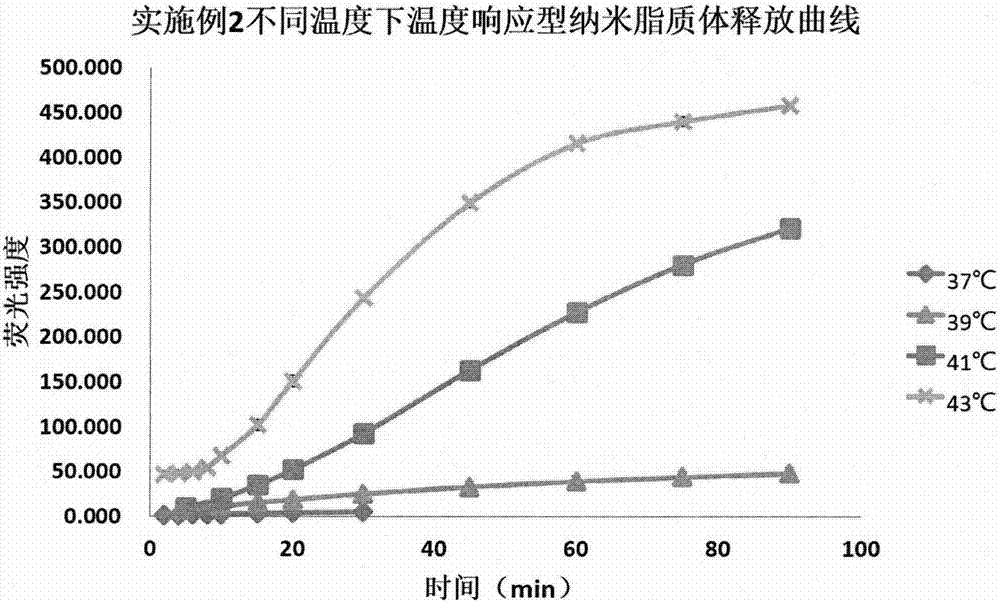
Embodiment 2
[0039] prescription:
[0040] Carboxyfluorescein 6mg
[0041] Dipalmitoylphosphatidylcholine 107.50mg
[0042] Stearoyl lysolecithin 12.50mg
[0043] Preparation:
[0044] 6 mg of carboxyfluorescein was added to 6 mL of pH 6.5 phosphate buffer, sonicated in the dark until dissolved, and set aside. 107.50 mg of dipalmitoyl phosphatidyl choline and 12.50 mg of stearoyl lysolecithin were added to 12 mL of a mixed solution of chloroform and methanol (3:1 by volume), and sonicated for half a minute. After the complete dissolution, put it on a rotary evaporator at 50°C and remove the organic solvent under reduced pressure. After drying, use the prepared 6mL carboxyfluorescein solution to hydrate for 1h at 50°C in the dark. The solution obtained after hydration was placed in a probe sonicator, and the power was 190W for 10 minutes. The ultrasonic mode was turned on for 3s and stopped for 4s. The solution obtained after sonication was extruded through a 0.22 μm filter to obtain the...
Embodiment 3
[0046] prescription:
[0047] Carboxyfluorescein 4mg
[0048] Dipalmitoylphosphatidylcholine 71.01mg
[0049] Stearoyl lysolecithin 8.99mg
[0050] Preparation:
[0051] 4 mg of carboxyfluorescein was added to 4 mL of phosphate buffer at pH 6.5, sonicated in the dark until dissolved, and set aside. 71.01 mg of dipalmitoyl phosphatidyl choline and 8.99 mg of stearoyl lysolecithin were added to 8 mL of a mixed solution of chloroform and methanol (3:1 by volume), and sonicated for half a minute. After the complete dissolution, put it on a rotary evaporator at 50°C and remove the organic solvent under reduced pressure. After drying, use the prepared 6mL carboxyfluorescein solution to hydrate for 1h at 50°C in the dark. The solution obtained after hydration was placed in a probe sonicator, and the power was 190W for 10 minutes. The ultrasonic mode was turned on for 3s and stopped for 4s. The solution obtained after sonication was extruded through a 0.22 μm filter to obtain the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com