Methyl N-dodecyl acetimidate and synthesis method thereof
A technology of methyl dodecyl acetimidate and a synthetic method, which is applied in the field of synthesis and organic synthesis, can solve the problems of complex reaction process, cumbersome operation, and no public literature report on the production method, and achieve high yield and high production efficiency. The process method is simple and the effect of filling the blank of production technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This embodiment provides a kind of N-dodecyl acetimidic acid methyl ester, which is synthesized through the following steps:
[0037] Weigh 1.89g of dodecylamine into a four-necked flask with a condenser, vacuum device and thermometer, add 20mL of methanol, and heat in a constant temperature oil bath until dodecylamine is completely dissolved;
[0038] When the temperature rises to 70°C, slowly drop 1.70g of N,N-dimethylacetamide dimethyl acetal into the solution, and react at 0.1MPa for 180min;
[0039] TLC (V CHCl3:CH3OH =3:1) sample climbing board, no raw material point occurs, indicating that dodecylamine is fully converted;
[0040] Reduce the pressure to 0.01MPa, and continue the reaction at 60°C for 30 minutes to obtain a light yellow liquid product, namely methyl N-dodecylglycolimate, the yield of methyl N-dodecylglycolimate was 71.4%.
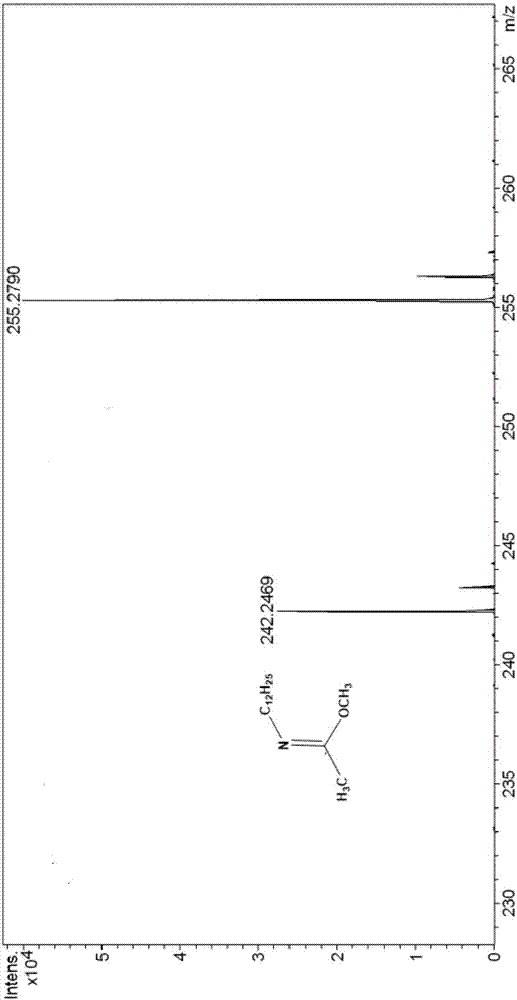
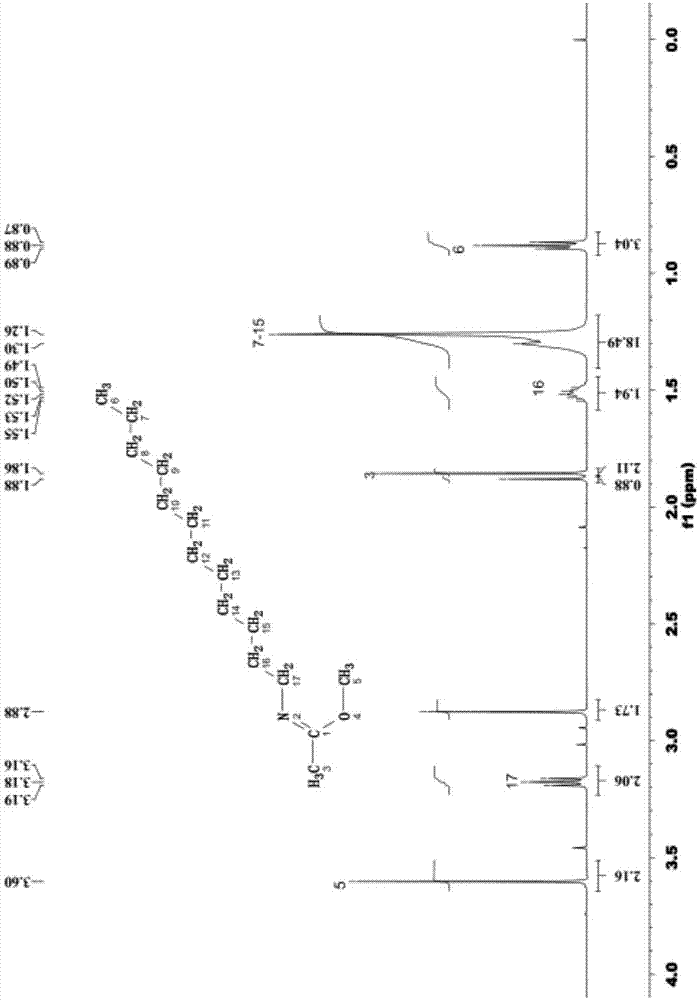
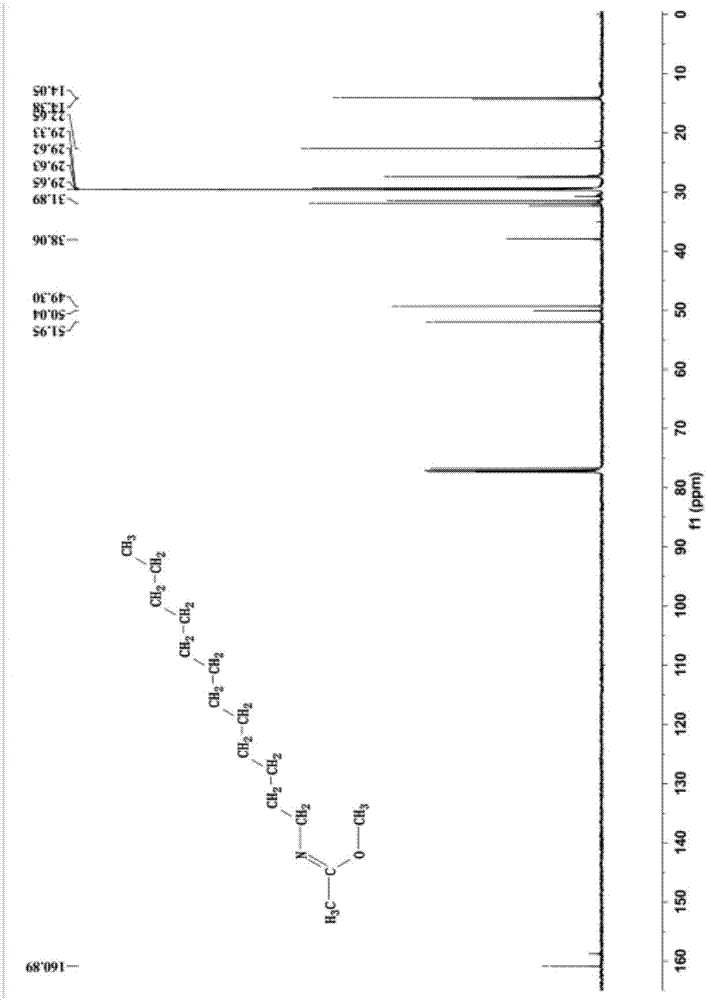
[0041] The mass spectrogram (diluted in acetonitrile) of the product synthesized in this embodiment is as follows: figure ...
Embodiment 2
[0046] This embodiment provides a kind of N-dodecyl acetimidic acid methyl ester, which is synthesized through the following steps:
[0047] Weigh 1.92g of dodecylamine into a four-necked flask with a condenser, a vacuum device and a thermometer, add 30mL of methanol, and heat in a constant temperature oil bath until dodecylamine is completely dissolved;
[0048] When the temperature rises to 40°C, slowly drop 1.60g of N,N-dimethylacetamide dimethyl acetal into the solution, and react for 10h at 0.1MPa;
[0049] TLC (V CHCl3:CH3OH =3:1) spotting climbs the board, no raw material point occurs, indicating that dodecylamine is fully converted;
[0050] Reduce the pressure to 0.03MPa, continue the reaction at 50°C for 1h, and obtain a light yellow liquid product, which is methyl N-dodecylglycolimate, the N-dodecylglycolimate methyl ester of this example The yield of ester was 72.2%.
[0051] The above examples illustrate that in the synthetic method of N-dodecylethylimidate met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com