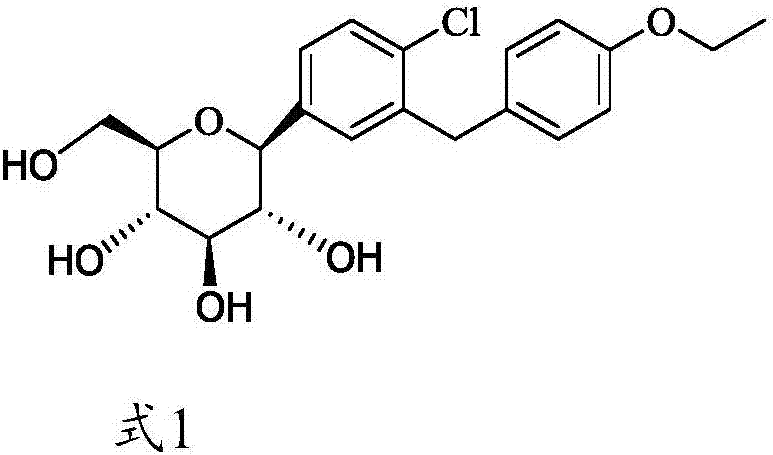
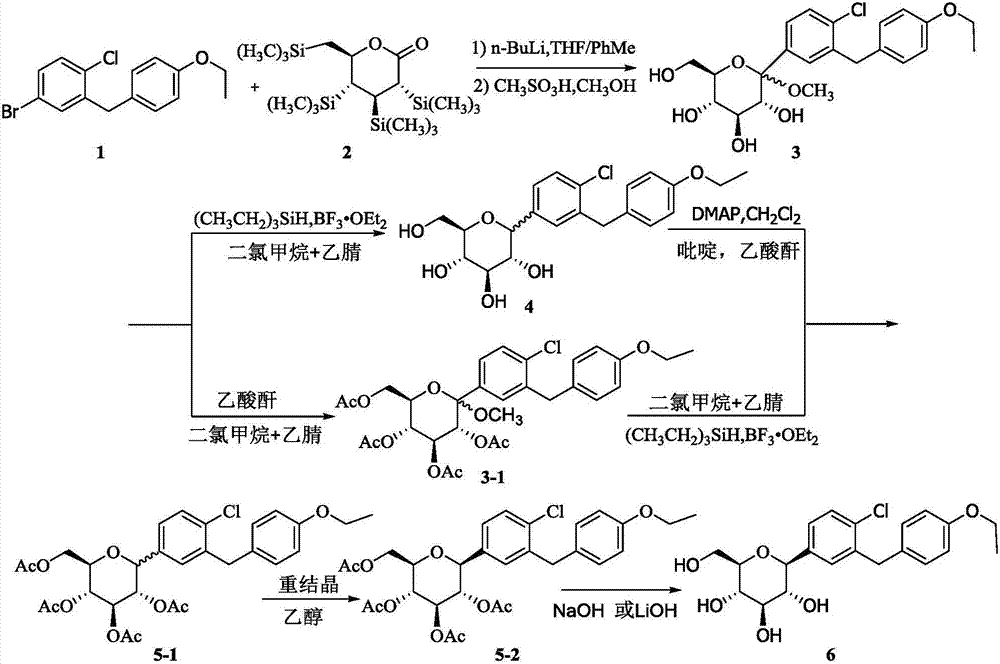
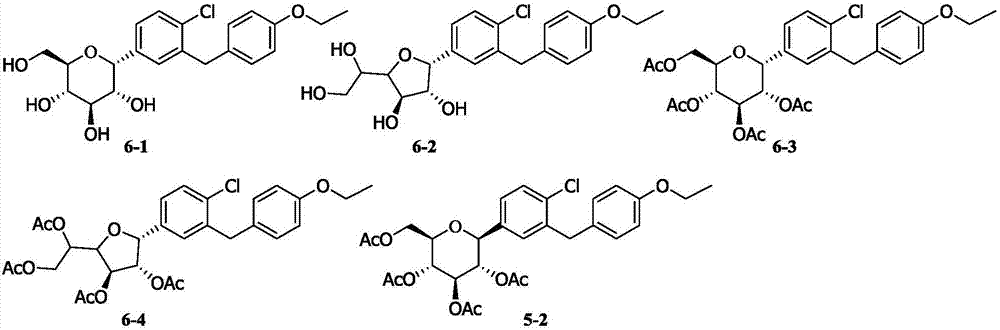
Method for synthesizing irregular glucitol
A synthesis method and technology of glucitol are applied in the synthesis field of pharmaceutical grade compounds, and can solve the problems of low total yield, long route, low yield and the like, and achieve the effects of mild reaction conditions, shortened reaction steps and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1. Preparation of amorphous (1S)-1,5-anhydro-1-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol
[0053] 1), the synthesis of compound 3
[0054] Under the protection of nitrogen, sequentially add toluene (500.0ml), tetrahydrofuran (400.0ml) and compound 1 (200.00g, 614.19mmol) into the reaction flask, cool to below -70°C with liquid nitrogen, and dropwise add 2.5M n-butyl Lithium n-hexane solution (270.25ml, 675.61mmol), after dripping, keep stirring for 10min, add dropwise the toluene solution (500.0ml) of compound 2 (344.10g, 737.03mmol), continue to keep stirring for 30-60min after dropping, TLC Monitor the response. After completion of the reaction, a solution of methanol (500.0 ml) of methanesulfonic acid (80.0 ml, 1.23 mol) prepared in advance was added dropwise, and reacted at 20-30° C. after the drop was completed. The reaction was monitored by TLC. After the reaction was completed, the pH of the reaction solution was adjusted to be ≥ 7 using sa...
Embodiment 2
[0063] Example 2. Preparation of amorphous (1S)-1,5-anhydro-1-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol (Dag net)
[0064] 1), the synthesis of compound 3
[0065] Under nitrogen protection, toluene (430.0ml), tetrahydrofuran (400.0ml) and compound 1 (200.00g, 614.19mmol) were successively added to the reaction flask, cooled to below -70°C with liquid nitrogen, and 2.5M n-butyl Lithium n-hexane solution (294.81ml, 737.03mmol), after dropping, keep stirring for 10min, add dropwise the toluene solution (370.0ml) of compound 2 (344.10g, 737.03mmol), continue to keep stirring for 30-60min after dropping, TLC Monitor the response. After the reaction was completed, a pre-prepared methanol (600.0 ml) solution of methanesulfonic acid (80.0 ml, 1.23 mol) was added dropwise, and reacted at 20-30° C. after the drop was completed. The reaction was monitored by TLC. After the reaction was completed, the pH of the reaction solution was adjusted to be ≥ 7 using saturated aque...
Embodiment 3
[0074] Example 3. Preparation of amorphous (1S)-1,5-anhydro-1-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol
[0075] 1), the synthesis of compound 3
[0076]Under the protection of nitrogen, sequentially add toluene (500.0ml), tetrahydrofuran (400.0ml) and compound 1 (200.00g, 614.19mmol) into the reaction flask, cool to below -70°C with liquid nitrogen, and dropwise add 2.5M n-butyl Lithium n-hexane solution (270.25ml, 675.61mmol), after dripping, keep stirring for 10min, add dropwise the toluene solution (500.0ml) of compound 2 (344.10g, 737.03mmol), continue to keep stirring for 30-60min after dropping, TLC Monitor the response. After completion of the reaction, a solution of methanol (500.0 ml) of methanesulfonic acid (80.0 ml, 1.23 mol) prepared in advance was added dropwise, and reacted at 20-30° C. after the drop was completed. The reaction was monitored by TLC. After the reaction was completed, the pH of the reaction solution was adjusted to be ≥ 7 using sat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com