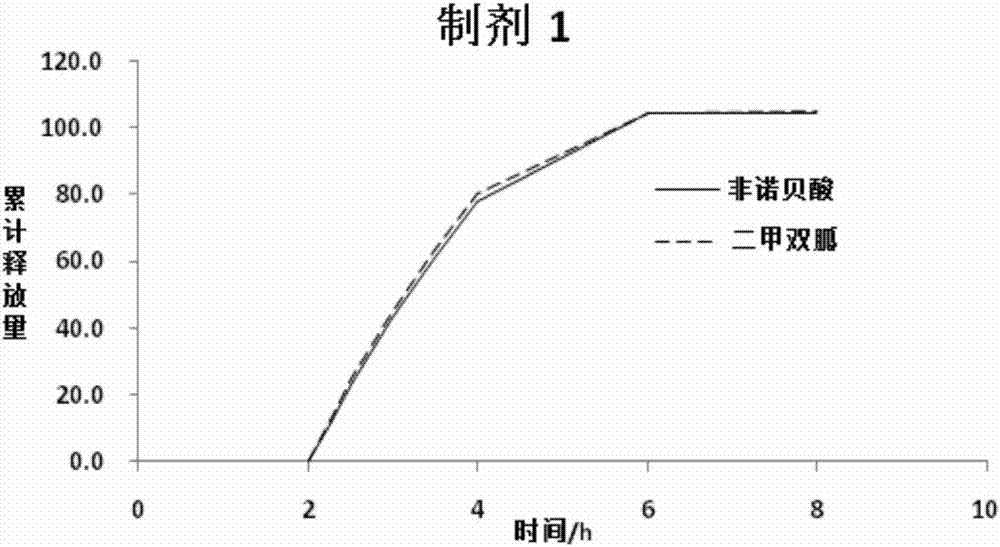
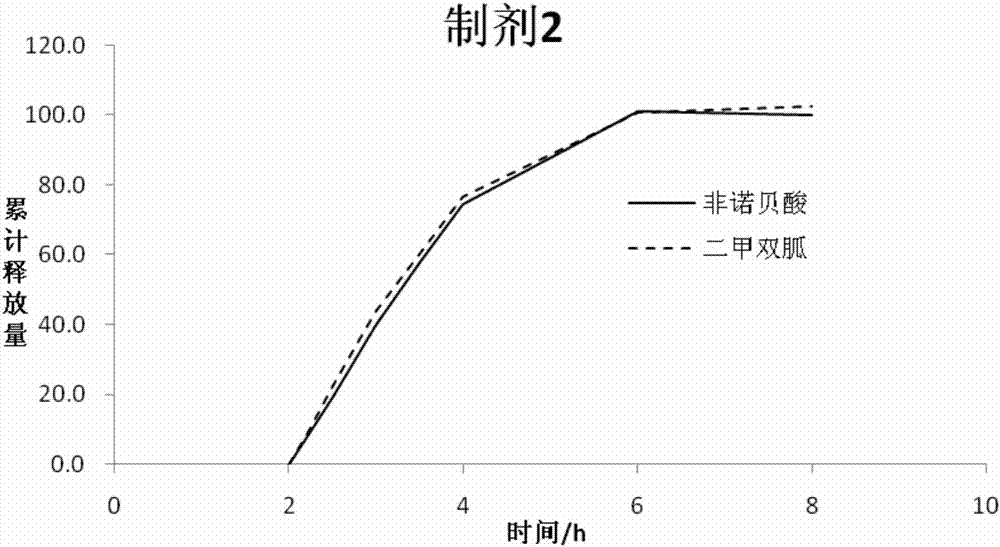
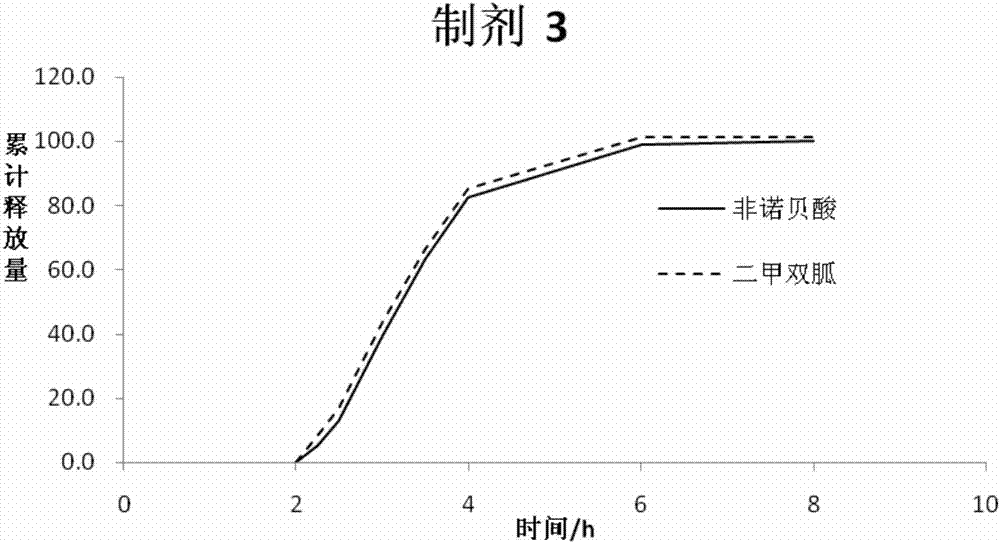
Metformin and fenofibric acid complex and preparation thereof
A technology of fenofibric acid and metformin, which is applied in the field of medicine, can solve the problems of poor tablet core quality, unfavorable clinical use, poor compressibility, etc., achieve a high qualification rate of preparations, avoid food effects and early release, and obviously Sustained release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of the complex of metformin and fenofibric acid
Embodiment 11
[0046] Take 12.9g of metformin and 31.9g of fenofibric acid (the molar ratio of the two is 1:1) and place it in a 500mL three-necked flask, add 250mL of isopropanol, heat to 80°C, and keep warm for 2h; concentrate under reduced pressure to make the reaction The volume of the liquid was reduced to about 100 mL, the temperature was lowered, and the crystallization was allowed to stand, and the crystals were collected by filtration and dried to obtain 36.2 g of the complex of metformin and fenofibric acid according to the present invention (white crystalline solid).
Embodiment 12
[0048] Get 19.4g of metformin and 31.9g of fenofibric acid (the molar ratio of the two is 1.5:1) and place in a 500mL three-necked flask, add 300mL of 95wt% ethanol, heat to 60°C, and keep warm for 2h; concentrate under reduced pressure to make the reaction The volume of the liquid was reduced to about 150 mL, the temperature was lowered, and the crystallization was allowed to stand, and the crystals were collected by filtration and dried to obtain 37.9 g of the complex of metformin and fenofibric acid according to the present invention (white crystalline solid).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com