Nicotinic acid riboside or nicotinamide riboside compositions, reduced derivatives thereof, and the use thereof
A kind of technology of compound and solvate, applied in nicotinic acid riboside or nicotinamide riboside composition, its reduced derivative and use field thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0122]Those skilled in the art will appreciate that the methods described are not the only means by which the compounds of the invention may be synthesized, and that a very wide range of synthetic organic reactions can potentially be used to synthesize the compounds of the invention. Those skilled in the art know how to choose and implement appropriate synthetic routes. Suitable synthetic methods can be identified by reference, including the following referenced sources such as: COMPREHENSIVE ORGANIC SYNTHESIS [Comprehensive Organic Synthesis] (eds. B.M. Trost & I. Fleming, Pergamon Press [Pergamon Press] 1991); COMPREHENSIVE ORGANIC FUNCTIONAL GROUPTRANSFORMATIONS [ COMPREHENSIVE ORGANIC FUNCTIONAL GROUP TRANSFORMATIONS II] (Editors A.R.Katritzky & R.J.K.Taylor, 2nd Edition, Elsevier [Elsevier] 2004); COMPREHENSIVE HETEROCYCLIC CHEMISTRY [Comprehensive Heterocyclic Chemistry] (edited by A.R. Katritzky & C.W. Rees, Pergamon Press [Pergamon Press] 1984); COMPREHENSIVE HETEROCYC...
example 1
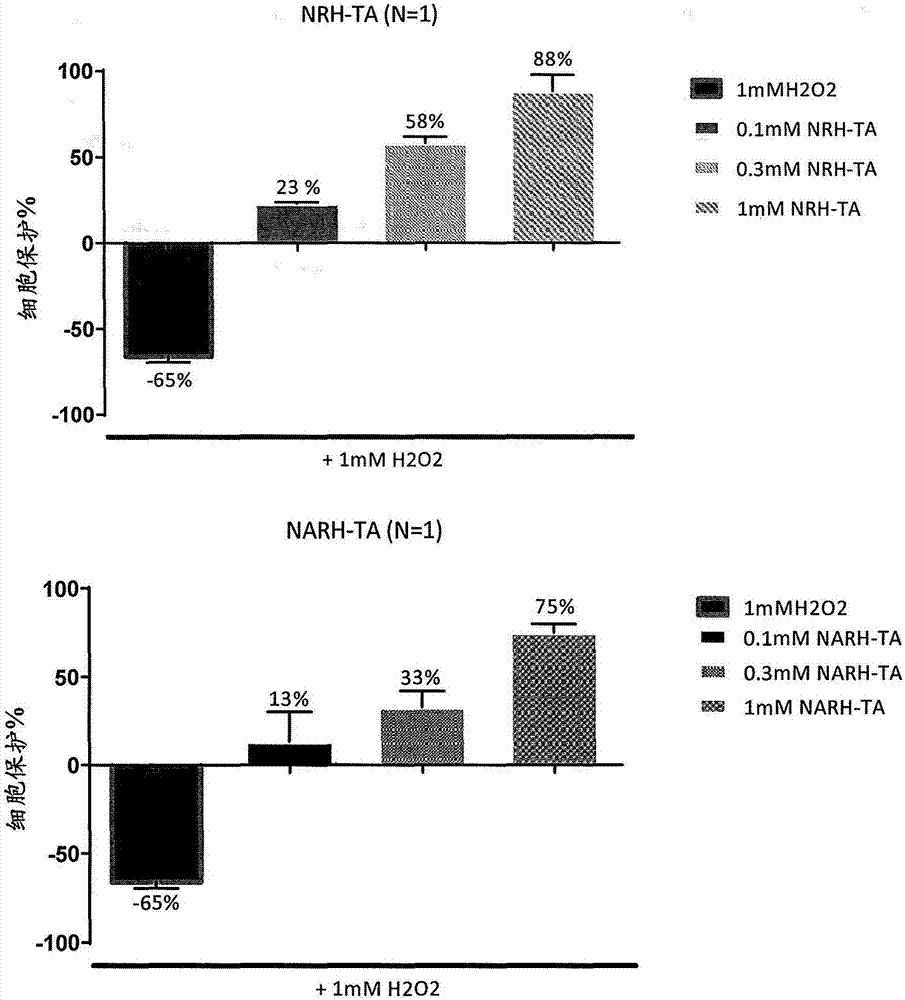
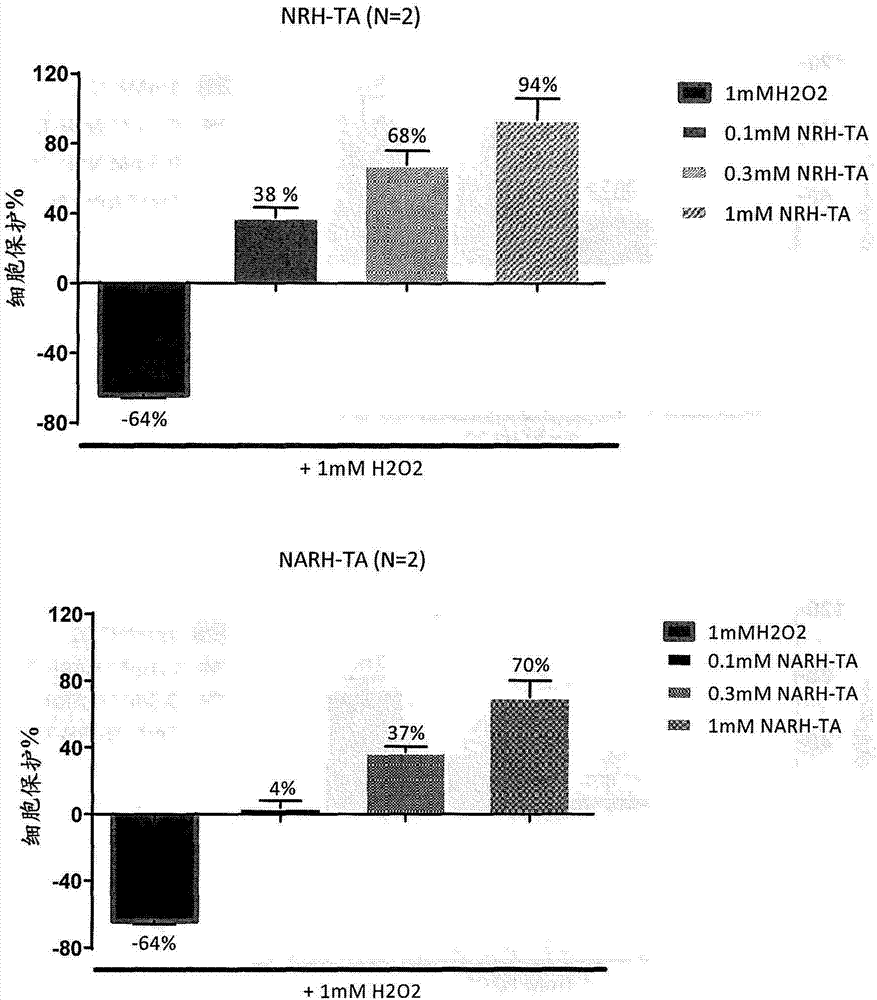
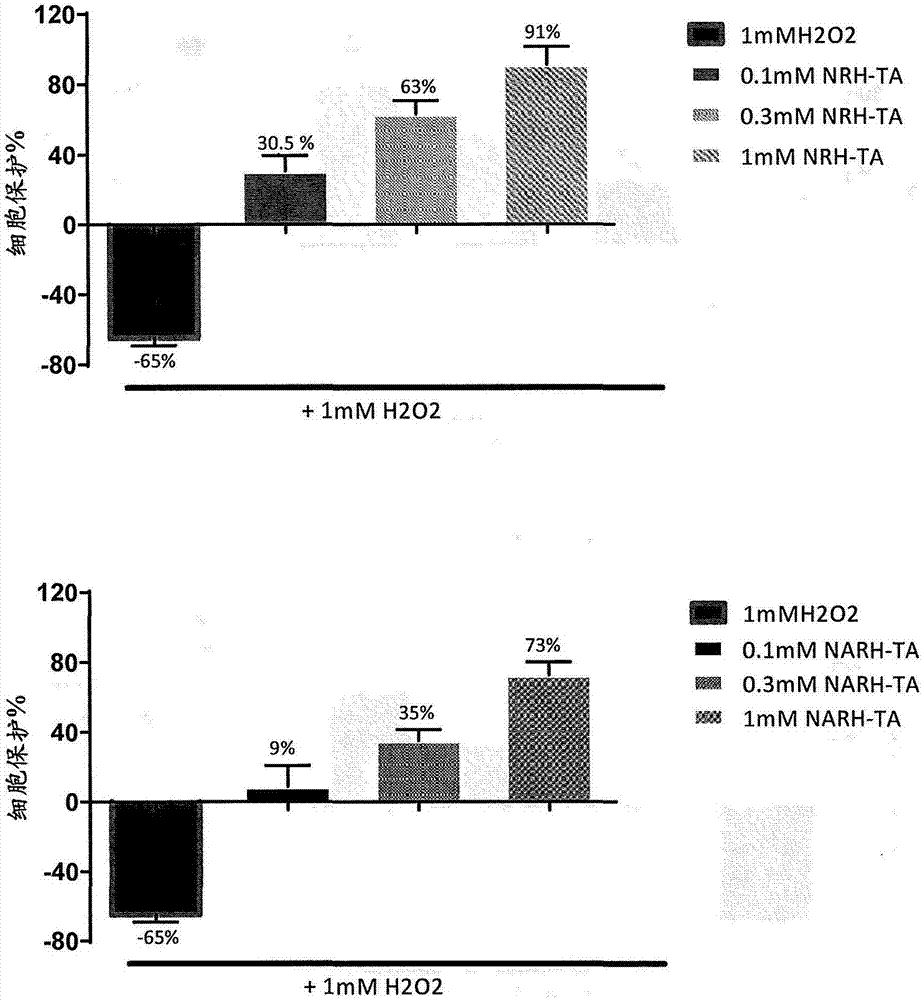
[0132] In one embodiment, one or more NR, NAR, NRH or NARH derivatives (including prodrugs or salts thereof) may be used as a vehicle for transdermal delivery of compounds and / or drug products. In a preferred embodiment, the derivative is selected from NRH triacetate (2) and NARH triacetate (4a).
[0133] In another embodiment, one or more NR, NAR, NRH or NARH derivatives (including prodrugs or salts thereof) may be used to: improve signs of aging, including superficial wrinkles, coarse deep wrinkles, enlarged pores , age spots, photodamage, scaling, flaking, dryness, sagging skin, puffy skin around the eyes, puffy skin around the lower cheeks, loss of elasticity of the skin, loss of firmness of the skin, loss of firmness of the skin, loss of barrier function, deformation of the skin from loss of resiliency, depigmentation, blotchiness, sallowness, hyperpigmentation, keratosis, hyperkeratosis, elastosis or collagen breakdown, and cellulite or combinations thereof. In a prefer...
example 2
[0137] In one embodiment, one or more NR, NAR, NRH or NARH derivatives (including prodrugs, solvates or salts thereof) may optionally be used in combination with pterostilbene. A useful dosage range for topical pterostilbene is from about 0.1% by weight to about 10% by weight, based on the total weight of the composition. Another suitable dosage range for topical pterostilbene is from about 1% to 2% by weight, based on the total weight of the composition. In a preferred embodiment, the derivative is selected from NRH triacetate (2) and NARH triacetate (4a). A useful dosage range for NRH triacetate (2) and NARH triacetate (4a) is about 0.1% to about 10% by weight, based on the total weight of the composition.
[0138] In this example, combinations containing NR, NAR, NRH or NARH act as UV-induced inflammatory modulators, affecting signs of aging and damage caused by, for example, UV / radiation including skin lightening, inflammation and sunburn redness.
[0139] Furthermore, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com