Covalent organic framework and preparation method thereof
A covalent organic framework and aldehyde compound technology, applied in the field of covalent organic framework and its preparation, can solve the problems of high cost, unfriendly environment and high risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093]
[0094] Add 2,4,6-triformylphloroglucinol (1.05g, 5.0mmol) and 80% hydrazine hydrate (470uL, 7.5mmol) into water (40mL) and sonicate for 5min, replace the argon three times under dry ice The tube is then sealed. After its temperature rose to room temperature, it was placed in an oven at 120° C. to react for 5 days. After the reaction was completed, it was cooled, filtered, washed with acetone, and dried in a vacuum oven to obtain 1.04 g of covalent organic framework material HCOF-1 as a dark red powder solid, with a yield of 94%.
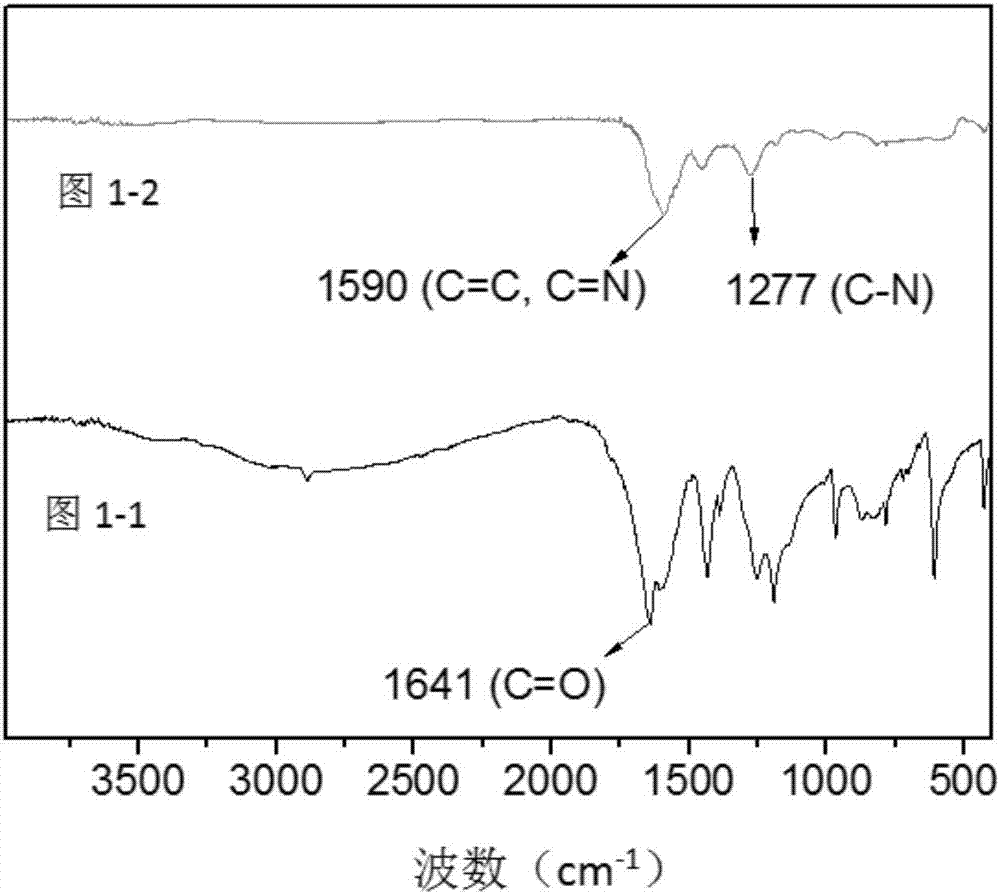
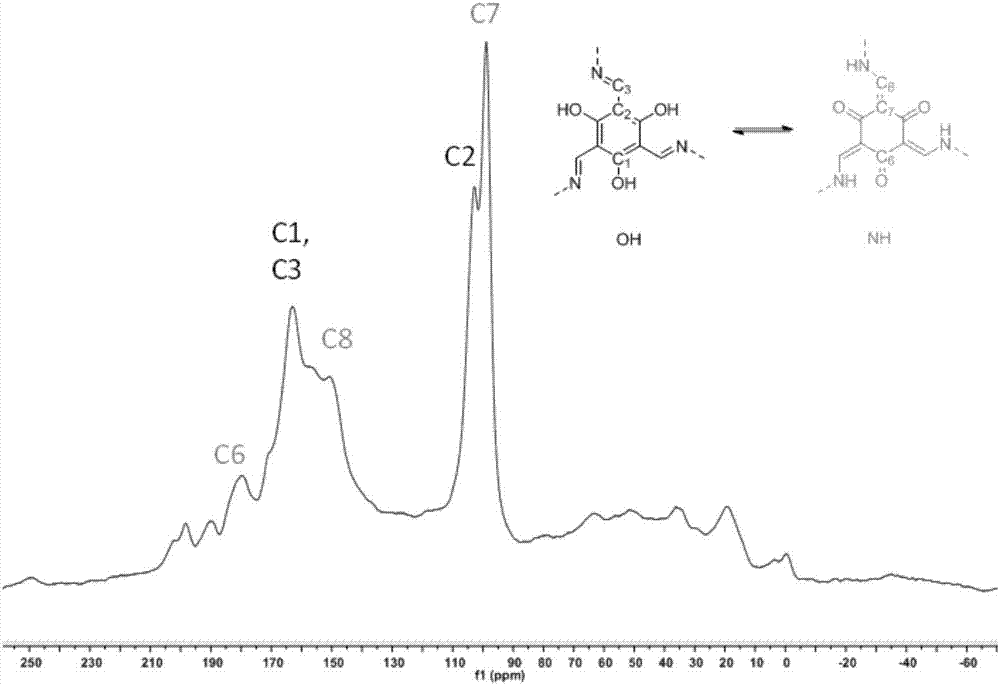
[0095] from figure 1 It can be found that the infrared spectrum of HCOF-1 ( Figure 1-2 ) and the infrared spectrum of raw material 2,4,6-triformylphloroglucinol ( Picture 1-1 ) is completely different, indicating that a reaction has occurred. The peak around 1590nm is the C=N bond shifting to the short wave due to the hydrogen bond, and superimposed with the C=C double bond peak of another isomer to form a broad peak, indicating the...
Embodiment 2
[0102]
[0103] Add trimellitic trialdehyde (1.0 g, 6.1 mmol) and 80% hydrazine hydrate (580 uL, 9.2 mmol) into water (40 mL) and sonicate for 5 min, add aqueous acetic acid (4 mL, 6 mol / L,) to the above cloudy solution, The tube was sealed after changing the argon gas three times under liquid nitrogen freezing. After its temperature rose to room temperature, it was placed in an oven at 120° C. to react for 5 days. After the reaction was completed, cooled, filtered, washed with acetone, and dried in a vacuum oven to obtain 0.93 g of light yellow fluffy solid covalent organic framework material (expressed as HCOF-2), yield: 86%.
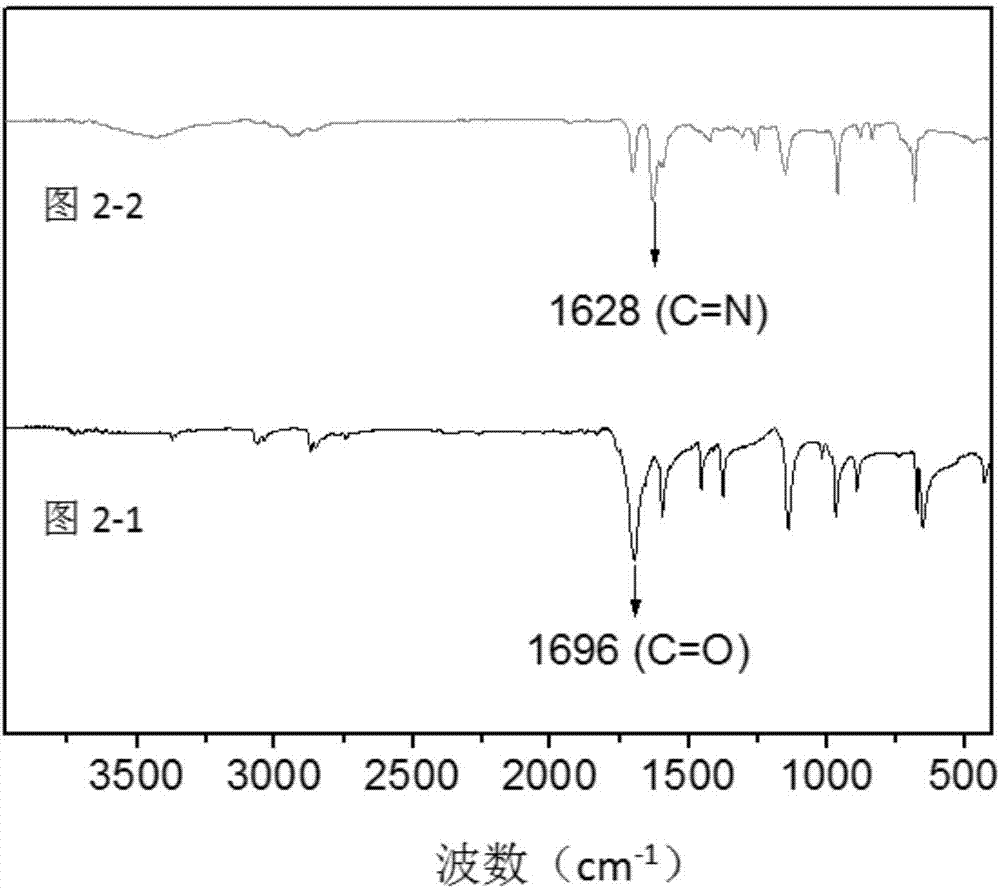
[0104] from figure 2 It can be found that the infrared spectrum of HCOF-2 ( Figure 2-2 ) and the infrared spectrum of the raw material pyromellitic trialdehyde ( diagram 2-1 ) diagrams are completely different, indicating that the reaction has occurred; in the infrared spectrum of HCOF-2, the vibration peak of C=N bond appears at 1628nm, whic...
Embodiment 3
[0110]Add 2,4,6-triformylphloroglucinol (1.05g, 5.0mmol) and 80% hydrazine hydrate (470uL, 7.5mmol) into water (40mL) and sonicate for 5min, pump and ventilate 3 times under refrigeration and seal this tube. After its temperature rose to room temperature, it was placed in an oven at 120°C to react for 3 days. After the reaction was completed, it was cooled, filtered, washed with acetone, and dried in a vacuum oven to obtain 1.0 g of covalent organic framework material HCOF-1 as a dark red powdery solid, with a yield of 91%. The characterization data of HCOF-1 obtained in this embodiment are basically the same as those in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com