Method for converting higher fatty acid into long-chain alkane by using photocatalysis decarboxylation method
A technology for higher fatty acids and long-chain alkanes, which is applied in the field of photocatalytic decarboxylation and conversion of higher fatty acids into long-chain alkanes. It can solve the problems of difficult realization and complicated reaction process, and achieve the effects of reduced reaction cost, simple preparation process and low energy consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
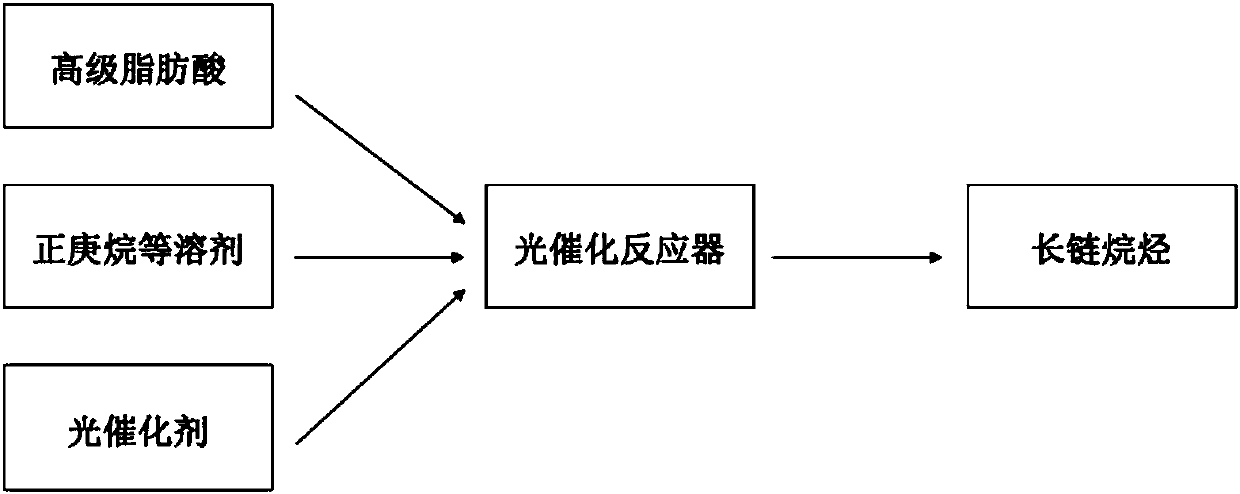
[0039] This embodiment provides a kind of photocatalyst TiO 2 A method for catalytically converting hexadecanoic acid to prepare pentadecane under the irradiation of an ultraviolet light source, such as figure 1 shown.
[0040] The method comprises the steps of:
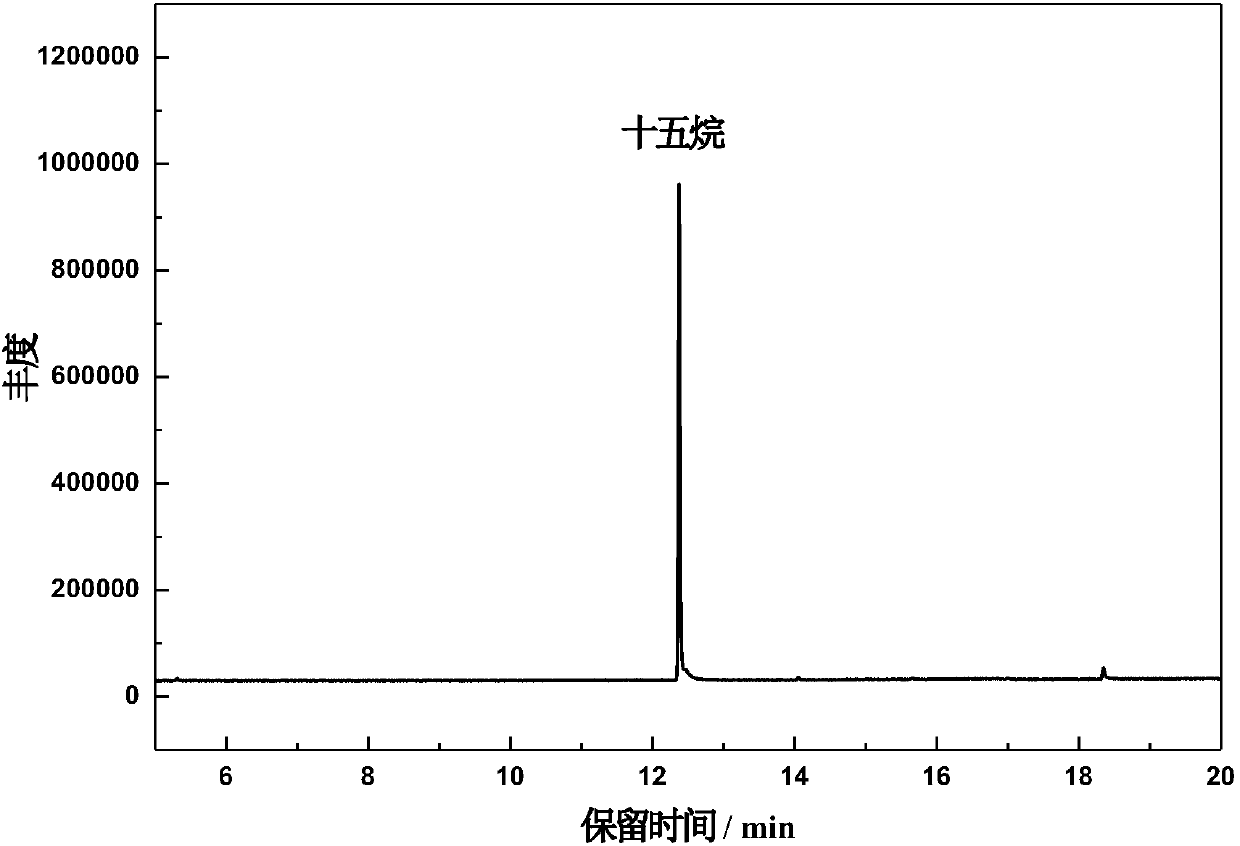
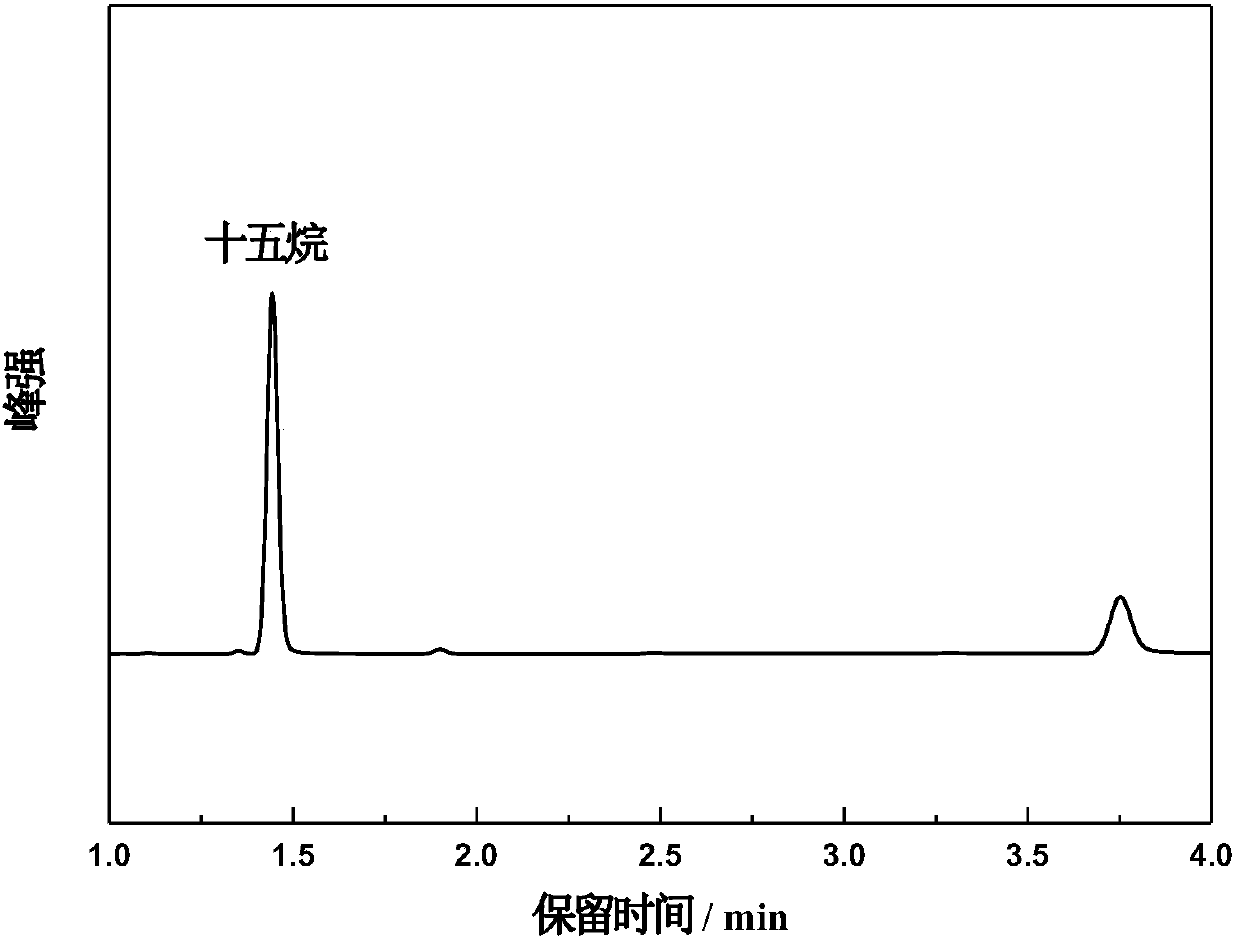
[0041] 30ml of n-heptane solution of hexadecanoic acid (concentration of hexadecanoic acid 0.005M) and TiO 2 Put 15 mg into the photocatalytic reactor, ultrasonicate for 30 minutes, purge the reaction device with nitrogen until the reactor is a nitrogen atmosphere, seal the reactor, and place it in a cooling circulating water tank at 20°C to control the reaction temperature at 20°C , and placed on a magnetic stirrer to stir, and reacted for 4h under the irradiation of a 150W mercury lamp. After the reaction, the product was characterized by GC-MS (see figure 2 ) and GC-FID quantitative analysis (see image 3 ), GC-MS qualitative analysis showed that pentadecane was the main product, and GC-FID quantitative ana...
Embodiment 2
[0045] In this example, the photocatalyst TiO 2 / Pt (1wt%) was used to catalyze the conversion of hexadecanoic acid to prepare pentadecane under the irradiation of ultraviolet light source.
[0046] The method of the present embodiment is as follows: adopt stainless steel photocatalytic reactor, 30ml (hexadecanoic acid concentration 0.005M) and TiO 2 / Pt (1wt%) 15mg was put into the photocatalytic reactor, ultrasonicated for 30min, and the reaction device was purged with nitrogen until the reactor was a nitrogen atmosphere, the reactor was sealed, and placed in a cooling circulating water tank at 20°C for use. The reaction temperature was controlled at 20°C, stirred on a magnetic stirrer, and reacted for 24 hours under the irradiation of a 150W mercury lamp. GC-MS qualitative analysis and GC-FID quantitative analysis were performed on the reacted product. GC-MS qualitative analysis showed that pentadecane was the main product, and GC-FID quantitative analysis showed that the ...
Embodiment 3
[0050] In this example, the photocatalyst TiO 2 / Co 3 o 4 (1wt%) catalytic conversion of hexadecanoic acid under the irradiation of ultraviolet light source to prepare pentadecane.
[0051] The method of the present embodiment is as follows: adopt stainless steel photocatalytic reactor, 30ml (hexadecanoic acid concentration 0.005M) and TiO 2 / Co 3 o 4 (1wt%) 15mg was put into the photocatalytic reactor, ultrasonicated for 30min, the reaction device was purged with nitrogen until the reactor was nitrogen atmosphere, the reactor was sealed, and it was placed in a 20°C cooling circulating water tank to make the reaction temperature Controlled at 20°C, placed on a magnetic stirrer for stirring, and reacted for 4 hours under the irradiation of a 150W mercury lamp. GC-MS qualitative analysis and GC-FID quantitative analysis were performed on the reacted product. GC-MS qualitative analysis showed that pentadecane was the main product, and GC-FID quantitative analysis showed that...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com