Process for preparing alcohol compounds with multiple chiral centers
An alcohol compound and compound technology, applied in the field of chiral compound synthesis, can solve the problem that chiral alcohol cannot obtain products with two or more chiral centers in one step, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Feeding: Add 30mg of the main raw material into a 10mL plastic bottle 100uL DMSO, 1.5mL phosphate buffer (100mmol / L, pH=7.0), the raw materials are evenly dispersed in the phosphate buffer;
[0034] (2) Ketoreductase: Add 1 mg coenzyme NAD+, 1.1 mg coenzyme NADP+, 130 mg D-glucose, 0.3 g ketoreductase main enzyme KRED and 5 mg glucose dehydrogenase GDH into a 10 mL plastic bottle, and the system pH=7.0;
[0035] (3) Reaction: The system was reacted at 30°C and stirred for 12 hours;
[0036] (4) Post-treatment: the system is extracted with 2 mL of ethyl acetate, left to separate liquids, and the product is detected by gas phase generation.
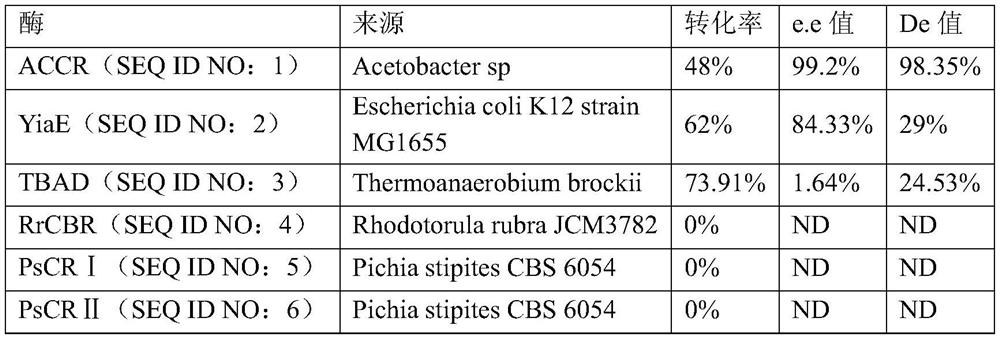
[0037]A total of 100 kinds of ketoreductases were screened (these 100 kinds of ketoreductases were all artificially synthesized from known sequences reported in the literature or the above sequences were artificially mutated), most of the reaction systems using the above ketoreductases left a large amount of raw materials , ...
Embodiment 2
[0041] (1) Feeding: Add 20g of the main raw material into the 2L reactor 500mL phosphate buffer (100mM, pH=6.0), the raw materials are evenly dispersed in the phosphate buffer;
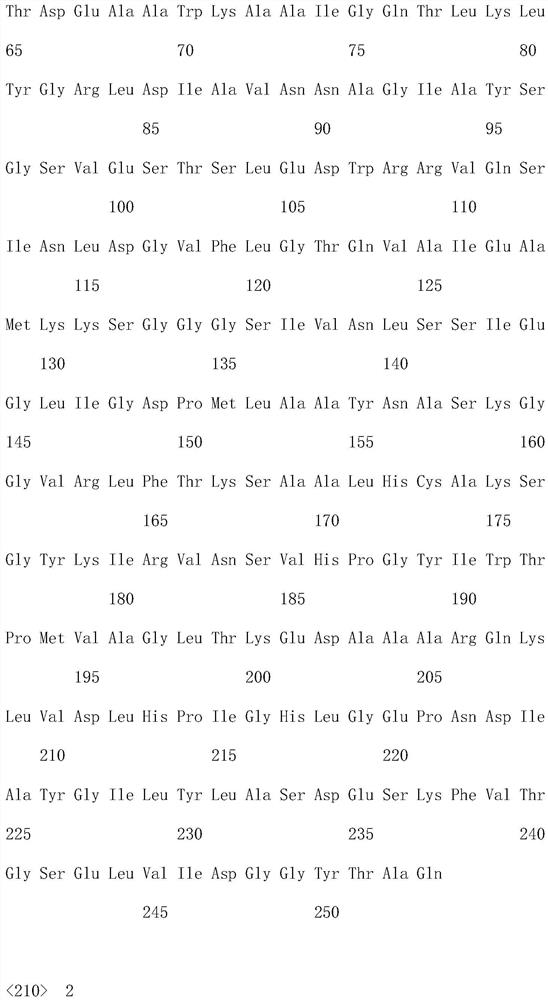
[0042] (2) Ketoreductase: Add 0.15g of coenzyme NAD+, 54g of D-glucose, 40g of ketoreductase ACCR (SEQ ID NO: 1) and 2g of glucose dehydrogenase GDH into a 2L reaction kettle, and the system pH= 6.0;
[0043] (3) Reaction: The system was reacted at 25°C and stirred for 24 hours;
[0044] (4) post-processing: the system is filtered with 200g diatomaceous earth, extracted with 1.8L ethyl acetate, left to separate liquids, the organic phase is dried, filtered, concentrated to obtain crude product, purified by column chromatography to obtain 8.2g of higher purity product The purity is 95.0%, the yield is 41%, the ee value is 99.5%, and the de value is 99.2%.
Embodiment 3
[0046] (1) Feeding: Add 20g of the main raw material into the 2L reactor 150mL isopropanol, 1L phosphate buffer (100mM, pH=7.5), the raw materials are evenly dispersed in the phosphate buffer;
[0047] (2) Ketoreductase: Add 0.2 g of coenzyme NAD+ and 10 g of ketoreductase ACCR (SEQ ID NO: 1) into a 2L reaction kettle, and the system pH=7.5;
[0048] (3) Reaction: The system was reacted at 40°C and stirred for 16 hours;
[0049] (4) post-processing: the system is filtered with 200g of diatomaceous earth, extracted with 1.5L of ethyl acetate, left to separate liquids, the organic phase is dried, filtered, concentrated to obtain a crude product, and purified by column chromatography to obtain 9.2g of higher purity product The purity is 92.7%, the yield is 46%, the ee value is 98.7%, and the de value is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com