Supramolecular intercalated structure light stabilizer and preparation method thereof
A technology of supramolecular intercalation and structured light, which is applied in the field of supramolecular intercalation structural light stabilizer and its preparation, can solve the problems of poor anti-light aging effect and achieve the purpose of overcoming poor anti-light aging effect and migration resistance Good, rich source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
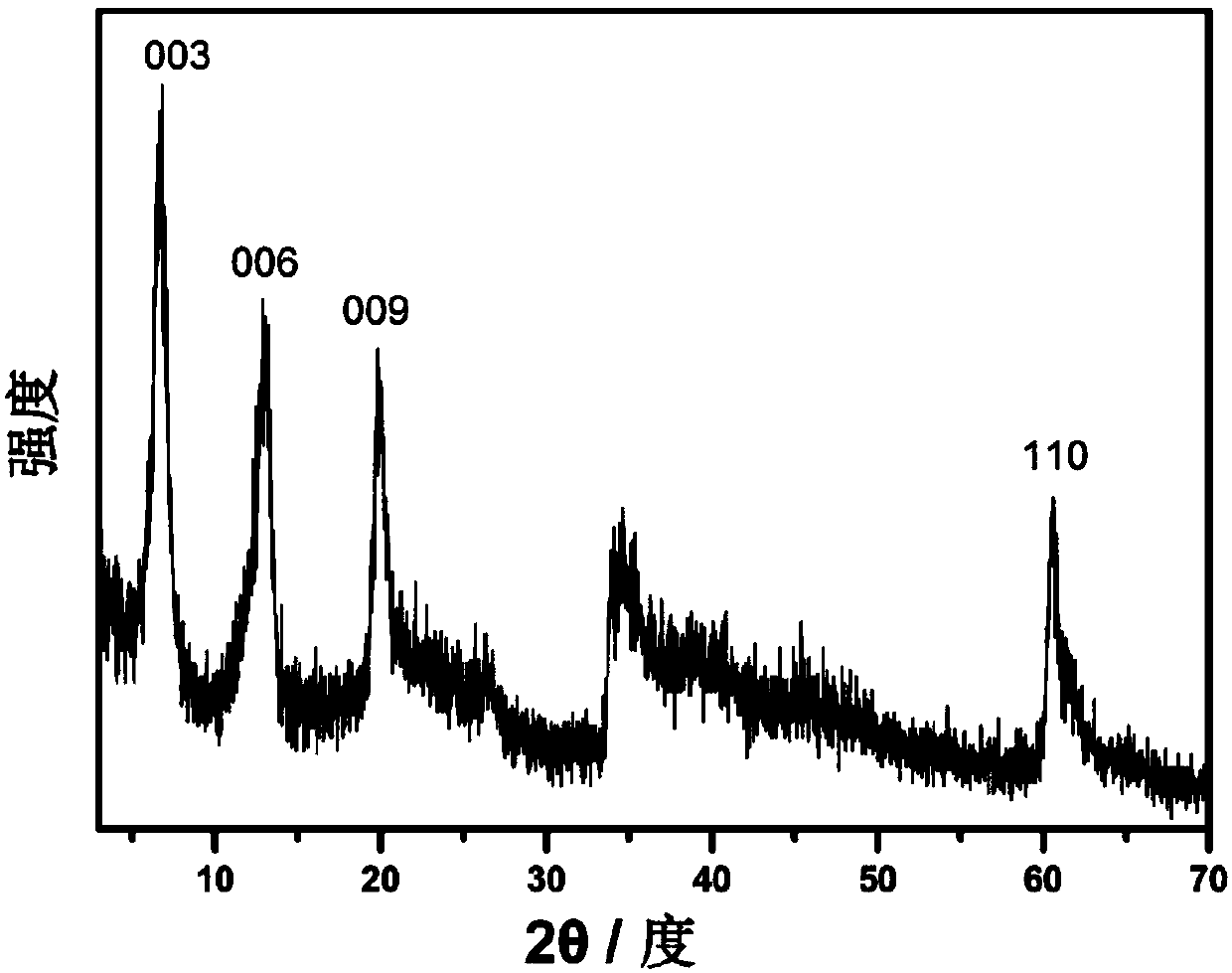
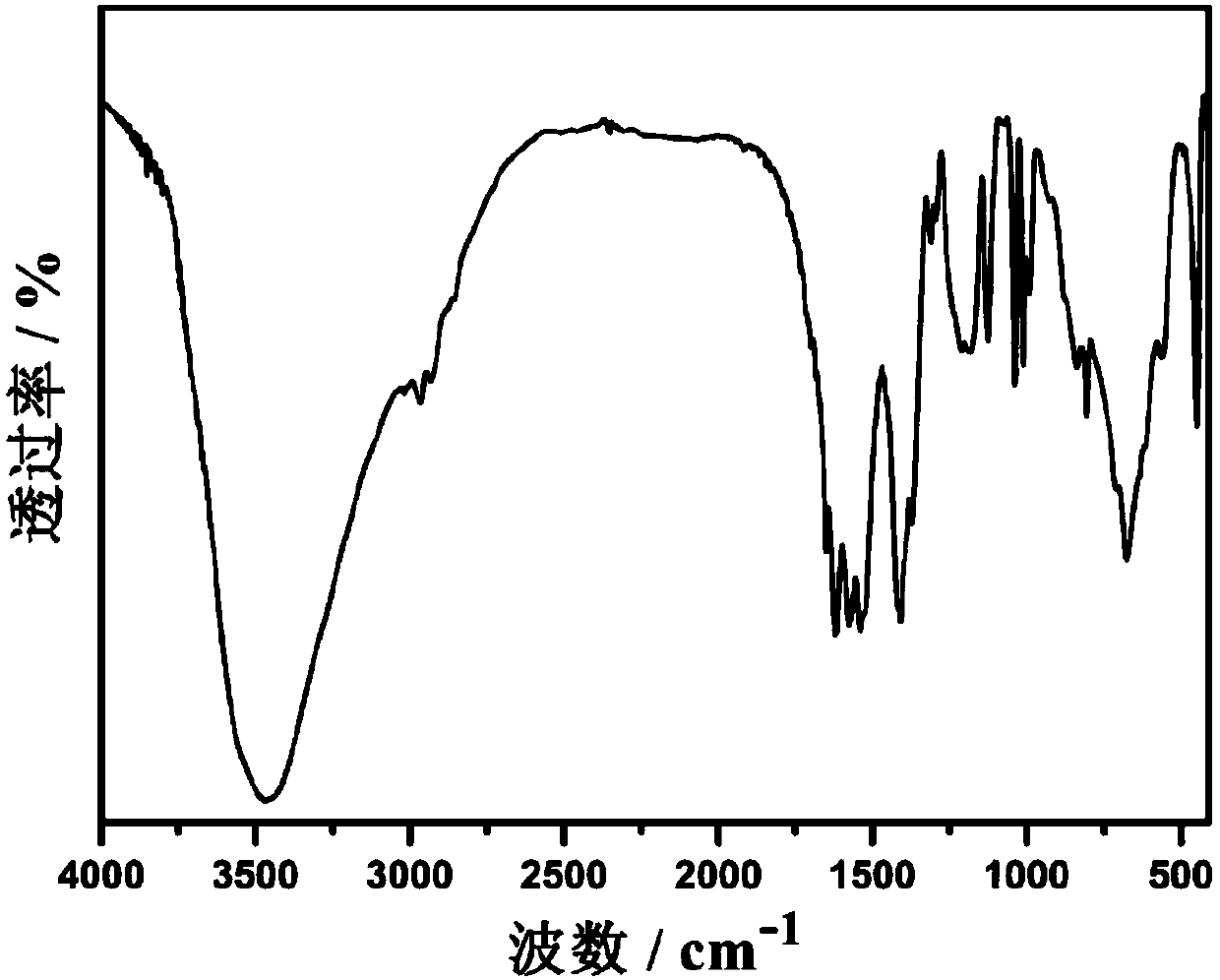
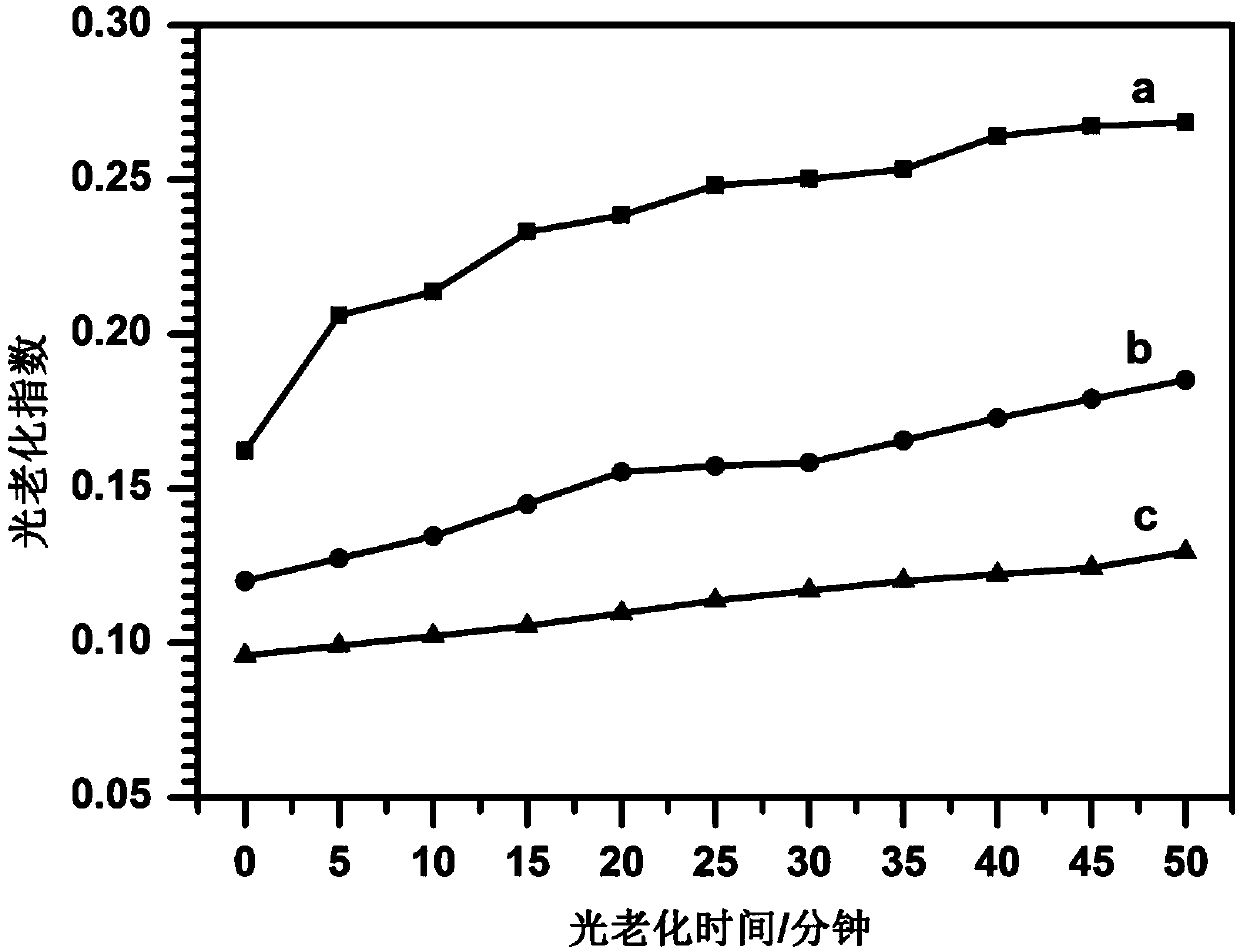
Image
Examples
Embodiment 1
[0036] Step A: Weigh 1.5g (5.0mmol) Mg(NO 3 ) 2 ·6H 2 O, 0.938g (2.5mmol) Al(NO 3 ) 3 9H 2 O, dissolved in 30ml to remove CO 2 salt solution in deionized water.
[0037] Step B: Dissolve 0.6g NaOH in 30ml of deionized water decarbonized to form an alkaline solution; weigh 0.5925g (2.5mmol) 2,2,6,6-tetramethylpiperidine-4-oxypropionic acid Dissolve sodium and 0.825g (2.5mmol) 2-hydroxy-4-methoxybenzophenone-5-sodium sulfonate in a mixed solvent composed of 40ml deionized water and ethylene glycol in addition to carbon dioxide, and add to the alkaline solution form a mixed solution.
[0038] Step C: Under nitrogen protection and stirring, mix and react the solutions prepared in steps A and B to form a precipitate, centrifuge and wash the formed precipitate 3 times, then add 120ml of deionized water to remove carbon dioxide to prepare a slurry, and weigh 0.0593g respectively (0.25 mmol) sodium 2,2,6,6-tetramethylpiperidine-4-oxypropionate and 0.0825 g (0.25 mmol) 2-hydrox...
Embodiment 2
[0040] Step A: Weigh 2.25g (7.5mmol) Mg(NO 3 ) 2 ·6H 2 O, 0.938g (2.5mmol) Al(NO 3 ) 3 9H 2 O, dissolved in 40ml to remove CO 2 salt solution in deionized water.
[0041] Step B: Dissolve 0.8g of NaOH in 45ml of deionized water decarbonized to form an alkaline solution; weigh 0.5925 g (2.5mmol) of 1,2,2,6,6-pentamethylpiperidine-4-oxyl Sodium acetate and 0.8 g (5 mmol) of sodium salicylate were dissolved in deionized water decarbonized and added to the alkaline solution to form a mixed solution.
[0042] Step C: Under nitrogen protection and stirring, mix and react the solutions prepared in steps A and B to form a precipitate, centrifuge and wash the formed precipitate 3 times, then add 120ml of deionized water to remove carbon dioxide to prepare a slurry, and weigh 0.0593g respectively (0.25 mmol) sodium 1,2,2,6,6-pentamethylpiperidine-4-oxyacetate and 0.04 g (0.25 mmol) sodium salicylate were added to the slurry, and then the precipitate was placed in a 95 °C water ba...
Embodiment 3
[0044] Step A: Weigh 3g (10.0mmol) Mg(NO 3 ) 2 ·6H 2 O, 0.938g (2.5mmol) Al(NO 3 ) 3 9H 2 O, dissolved in 45ml to remove CO 2 salt solution in deionized water.
[0045] Step B: Dissolve 1 g of NaOH in 25 ml of deionized water with carbon dioxide removed to form an alkaline solution. Weigh 1.175g (5mmol) sodium 2,2,6,6-tetramethylpiperidine-4-aminopropionate and 0.3978g (2.5mmol) sodium p-aminobenzoate and dissolve them in deionized water and ethylene glycol Alcohol mixed solvent, added to the alkali solution to form a mixed solution.
[0046] Step C: Under nitrogen protection and stirring, mix the solutions prepared in steps A and B to form a precipitate, centrifuge and wash the formed precipitate 3 times, then add 120ml of deionized water to remove carbon dioxide to prepare a slurry, and weigh 0.0588g respectively (0.25 mmol) sodium 2,2,6,6-tetramethylpiperidine-4-aminopropionate and 0.0398 g (0.25 mmol) sodium p-aminobenzoate were added to the slurry, and then the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com