Bimetallic compound with limited configuration as well as preparation method and application of bimetallic compound
A compound and bimetallic technology, applied in the field of configuration-limited bimetallic compounds and their preparation, can solve the problems of long synthesis route of catalyst and expensive raw materials, achieve good catalytic activity and copolymerization ability, easy separation and purification, and short synthesis route. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
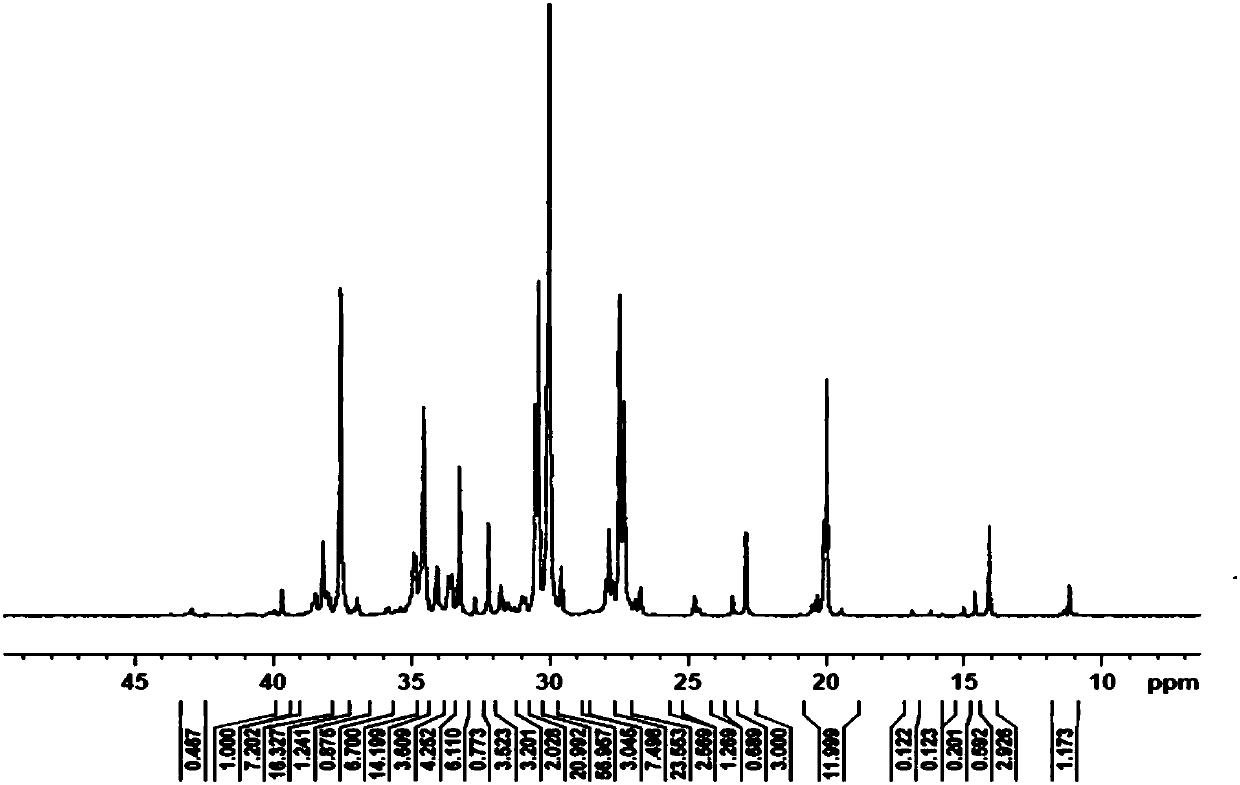
Embodiment 1
[0058] This embodiment provides a bimetallic compound in a restricted configuration, the molecular formula of the complex C1 is: [(o-O(C 6 h 4 )CH=N)(TiCl 3 )] 2 (p-C 6 h 4 ).
[0059] The synthetic route of complex C1 is as follows:
[0060]
[0061] The specific preparation process of complex C1 is:
[0062] (1) Under argon protection, a reflux condenser was connected to a 250mL three-necked flask equipped with a magnetic stirrer, and at room temperature, salicylaldehyde (4.88g, 0.04mol), p-phenylenediamine (2.16 g, 0.02mol) and 120mL ethanol, 1mL methanesulfonic acid, heated to reflux for 6-8h, an orange-yellow solid was produced. Stop stirring, return to room temperature, suction filter, wash the solid with ethanol, and dry to obtain orange-yellow solid L1, 5.82 g, yield 92.0%.
[0063] 1 H NMR (400MHz, d-DMSO, ppm) δ9.03 (s, 1H, -CH=N); 13.07 (s, 1H, -OH); 6.98 (d, J = 8.4Hz, 1H, Ar-H) ;7.00(t,J=7.6Hz,1H,Ar-H);7.43(t,J=7.6Hz,1H,Ar-H);7.67(d,J=7.2Hz,1H,Ar-H);7...
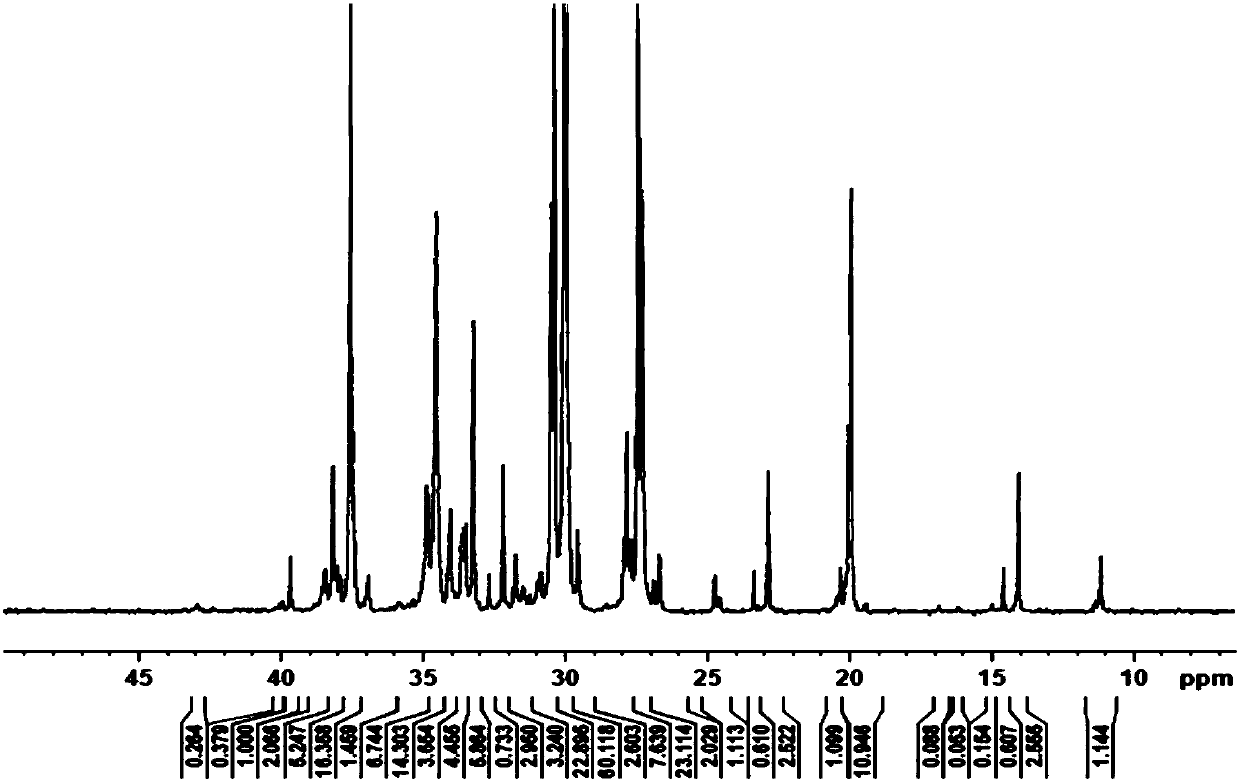
Embodiment 2
[0068] The present embodiment provides a bimetallic compound in a restricted configuration, and the molecular formula of the complex C2 is: [(o-O(C 6 h 3 )-3-Me)CH=N)(TiCl 3 )] 2 (p-C 6 h 4 ).
[0069] The preparation process of complex C2 is the same as the preparation process of complex C1 in Example 1, the difference is only that salicylaldehyde is replaced by 3-allyl salicylaldehyde, and methanesulfonic acid is replaced by benzenesulfonic acid. Yield 76.0%.
[0070] 1 H NMR (400HMz, DMSO): δ9.07(s, 1H, -CH=N); 2.23(s, 3H, -CH 3 ); 6.91(t,1H,Ar-H); 7.33(d,1H,Ar-H); 7.53(d,1H,Ar-H); 7.60(s,2H,Ar-H). 13 C NMR (100HMz, DMSO): δ163.76, 158.94, 145.90, 134.56, 130.62, 125.31, 122.62, 118.80, 118.27, 15.31. Anal. Calcd for C 22 h 18 Cl 6 N 2 o 2 Ti 2 :C,43.20;H,3.62;N,3.87.Found:C,43.19;H,4.02;N,3.46.IR.(KBr,cm -1 )3330.9(s),1638.3(s),1597.6(s),1560.1(m),1516.3(w),1491.5(w),1384.7(m),1269.4(m),1190.8(m),873.9(m ), 894.7(m), 864.6(m), 783.8(w), 742.2(w), 428.3(w). ...
Embodiment 3
[0072] The present embodiment provides a bimetallic compound in a restricted configuration, and the molecular formula of the complex C3 is: [(o-O(C 6 h 3 )-3-allyl)CH=N)(TiCl 3 )] 2 (p-C 6 h 4 ).
[0073] The preparation process of complex C3 is the same as the preparation process of complex C1 in Example 1, the difference is only that salicylaldehyde is replaced by 3-allyl salicylaldehyde, and methanesulfonic acid is replaced by p-toluenesulfonic acid. Yield 59.6%.
[0074] 1H NMR (400HMz, DMSO): δ9.07(s, 1H, -CH=N); 3.41(d, 2H, -CH 2 ); 5.05(m,1H,=CH a ); 5.08(m,1H,=CH b ); 6.01(m,1H,=CH); 6.95(t,1H,Ar-H); 7.30(d,1H,Ar-H); 7.54(d,1H,Ar-H); 7.59(s,2H ,Ar-H). 13 C NMR (100HMz, DMSO): δ163.80, 158.48, 145.95, 136.50, 133.70, 131.17, 127.39, 122.64, 118.95, 118.60, 115.86, 33.10. 26 h 22 Cl 6 N 2 o 2 Ti 2 : C, 45.10; H, 4.33; N, 3.01.Found: C, 45.57; H, 4.30; N, 3.04.IR.(KBr, cm -1 )3359.9(m),1637.8(s),1595.3(s),1556.8(s),1491.4(w),1437.3(w),1385.2(m),1245.0(w)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com