A kind of constrained configuration double metal compound and its preparation method and application
A compound and bimetallic technology, which is applied in the field of constrained configuration bimetallic compounds and their preparation, can solve the problems of long catalyst synthesis route and expensive raw materials, achieve good catalytic activity and copolymerization ability, easy separation and purification, and simple synthesis process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
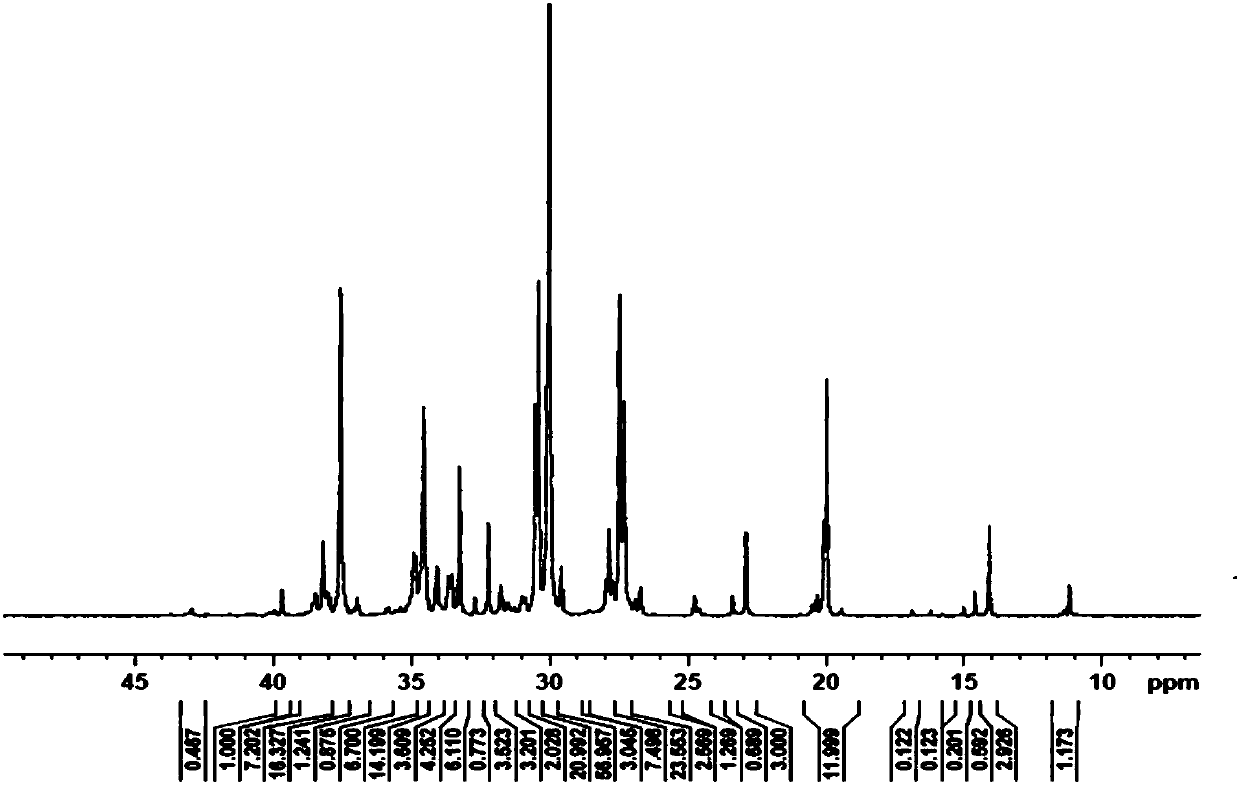
[0058] This example provides a constrained configuration bimetallic compound, and the molecular formula of the complex C1 is: [(o-O(C 6 H 4 )CH=N)(TiCl 3 )] 2 (p-C 6 H 4 ).
[0059] The synthetic route of complex C1 is as follows:
[0060]
[0061] The specific preparation process of complex C1 is:
[0062] (1) Under the protection of argon, a reflux condenser was attached to a 250mL three-necked flask equipped with a magnetic stirrer, and at room temperature, salicylaldehyde (4.88g, 0.04mol), p-phenylenediamine (2.16 g) were added to the flask. g, 0.02mol) and 120mL of ethanol, 1mL of methanesulfonic acid, heated to reflux for 6-8h, an orange-yellow solid was produced. Stop stirring, return to room temperature, filter with suction, wash the solid with ethanol, and dry to obtain an orange-yellow solid L1, 5.82 g, with a yield of 92.0%.
[0063] 1 H NMR (400MHz, d-DMSO, ppm) δ 9.03 (s, 1H, -CH=N); 13.07 (s, 1H, -OH); 6.98 (d, J=8.4Hz, 1H, Ar-H) ;7.00(t,J=7.6Hz,1H,A...
Embodiment 2
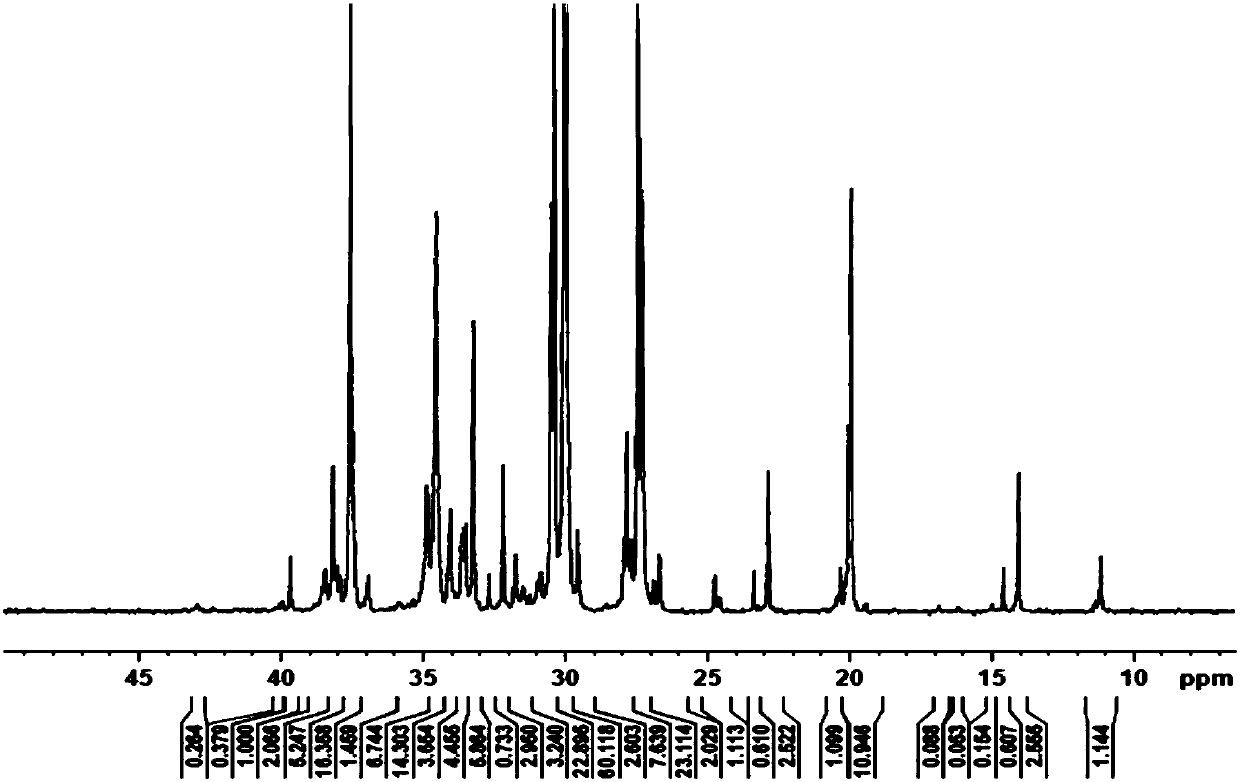
[0068] This embodiment provides a constrained configuration bimetallic compound, and the molecular formula of the complex C2 is: [(o-O(C 6 H 3 )-3-Me)CH=N)(TiCl 3 )] 2 (p-C 6 H 4 ).
[0069] The preparation process of complex C2 is the same as the preparation process of complex C1 in Example 1, except that 3-allyl salicylaldehyde is used instead of salicylaldehyde, and benzenesulfonic acid is used instead of methanesulfonic acid. Yield 76.0%.
[0070] 1 H NMR (400HMz, DMSO): δ 9.07 (s, 1H, -CH=N); 2.23 (s, 3H, -CH 3 ); 6.91(t, 1H, Ar-H); 7.33(d, 1H, Ar-H); 7.53(d, 1H, Ar-H); 7.60(s, 2H, Ar-H). 13 C NMR (100HMz, DMSO): δ163.76, 158.94, 145.90, 134.56, 130.62, 125.31, 122.62, 118.80, 118.27, 15.31.Anal.Calcd for C 22 H 18 Cl 6 N 2 O 2 Ti 2 :C,43.20;H,3.62;N,3.87.Found:C,43.19;H,4.02;N,3.46.IR.(KBr,cm -1 )3330.9(s), 1638.3(s), 1597.6(s), 1560.1(m), 1516.3(w), 1491.5(w), 1384.7(m), 1269.4.(m), 1190.8(m), 873.9(m) ), 894.7(m), 864.6(m), 783.8(w), 742.2(w), 428.3(w)....
Embodiment 3
[0072] This embodiment provides a constrained configuration bimetallic compound, and the molecular formula of the complex C3 is: [(o-O(C 6 H 3 )-3-allyl)CH=N)(TiCl 3 )] 2 (p-C 6 H 4 ).
[0073] The preparation process of complex C3 is the same as the preparation process of complex C1 in Example 1, except that 3-allyl salicylaldehyde is used instead of salicylaldehyde, and p-toluenesulfonic acid is used instead of methanesulfonic acid. Yield 59.6%.
[0074] 1H NMR (400HMz, DMSO): δ9.07(s, 1H, -CH=N); 3.41(d, 2H, -CH 2 ); 5.05(m,1H,=CH a ); 5.08(m,1H,=CH b ); 6.01(m,1H,=CH); 6.95(t,1H,Ar-H); 7.30(d,1H,Ar-H); 7.54(d,1H,Ar-H); 7.59(s,2H ,Ar-H). 13 C NMR (100HMz, DMSO): δ163.80, 158.48, 145.95, 136.50, 133.70, 131.17, 127.39, 122.64, 118.95, 118.60, 115.86, 33.10. 26 h 22 Cl 6 N 2 O 2 Ti 2 : C, 45.10; H, 4.33; N, 3.01.Found: C, 45.57; H, 4.30; N, 3.04.IR.(KBr, cm -1 )3359.9(m),1637.8(s),1595.3(s),1556.8(s),1491.4(w),1437.3(w),1385.2(m),1245.0(w),1148.7(w),1012.5(w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com